Somatostatin
SS
SST
SOM
Octreotide (synthetic)
GROWTH HORMONE INHIBITING HORMONE (GHIH)
Contents
Most Frequent Uses:
- Growth hormone tumors
- Acromegaly
- Carcinoid tumors
- Vasoactive Intestinal Peptide tumors (VIPomas)
- Treatment of intestinal, biliary and pancreatic fistulae
- Treat acute bleeding from esophageal varices, gastrointestinal ulcers, and gastritis
- Symptomatic treatment of excessive secretion from endocrine tumors of the gastrointestinal tract
- Treatment of postoperative complications and prophylaxis following pancreatic surgery
- Correct impaired glucagon secretion in response to hypoglycemia in diabetes mellitus.
- Coadjuvant treatment in diabetic ketoacidosis
Dosage:
- SQ – none established
Safety and Potential Side Effects/Contraindications:
- Somatostatin and analogs are reported safe in recommended dosages.
- Contraindicated in:
- Hypersensitivity to any component of the product
- Complicated cardiovascular patients
- Biliary issues
- Pregnancy
- Safety in pediatrics has not been established
- The following adverse effects have been reported:[i]
- Gastrointestinal abnormalities (34% to 61%) are transient and usually mild to moderate. They include diarrhea, nausea, abdominal discomfort, gallbladder abnormalities, such as cholelithiasis and microlithiasis, biliary sediment and sludge due to alteration of fat absorption, and possibly by decreasing motility of the gallbladder. Gallbladder alterations and biliary stones reported in up to 60% cases treated with somatostatin receptor lignans (SRLs) on a LONG-TERM (> 48 months) basis; ursodeoxycholic acid is recommended as treatment and also support from biliary supplements such as Globe artichoke (Cynara scolymus) leaf 600mg twice daily (std. to 13-18% caffeoylquinic acids)[ii]
- Bradycardia occurs in 25% of patients with acromegaly, conduction abnormalities (10%), and arrhythmias (9%).
- Hypoglycemia (3%) and hyperglycemia (16%) occur due to alteration in glucose metabolism, usually mild in severity.
- Hypothyroidism: In patients with acromegaly, subclinical hypothyroidism occurred in 12%, and goiter occurred in 6% during treatment with octreotide acetate. In patients without acromegaly, isolated cases of hypothyroidism have been reported. However, there was no incidence of goiter in this subset of patients.
- Dermatologic: Itching (18%).
- Pain at the injection site (7.7%).
- Headache and dizziness (6%).
- Other side effects, documented in less than 4% of patients on octreotide, include cold and flu symptoms, weakness, fatigue, depression, blurred vision, pruritus, hair loss, vision disturbance, flushing of the skin, arthralgia, lower back pain, increased urinary frequency especially during waking hours, and urinary tract infection, edema, bruising and hematoma formation at the injection site, and malabsorption of fat and other nutrients.
- Concomitant administration of any form of sugar (including glucose and fructose solutions or TPN) favors glycemic disturbances and requires a closer monitoring of blood sugar. Administration of insulin may be required.
[i] Borna RM, Jahr JS, Kmiecik S, Mancuso KF, Kaye AD. Pharmacology of Octreotide: Clinical Implications for Anesthesiologists and Associated Risks. Anesthesiol Clin. 2017 Jun;35(2):327-339.
[ii] Prencipe N, et al. Biliary adverse events in acromegaly during somatostatin receptor ligands: predictors of onset and response to ursodeoxycholic acid treatment. Pituitary. 2021;24(2):242-51.
Description
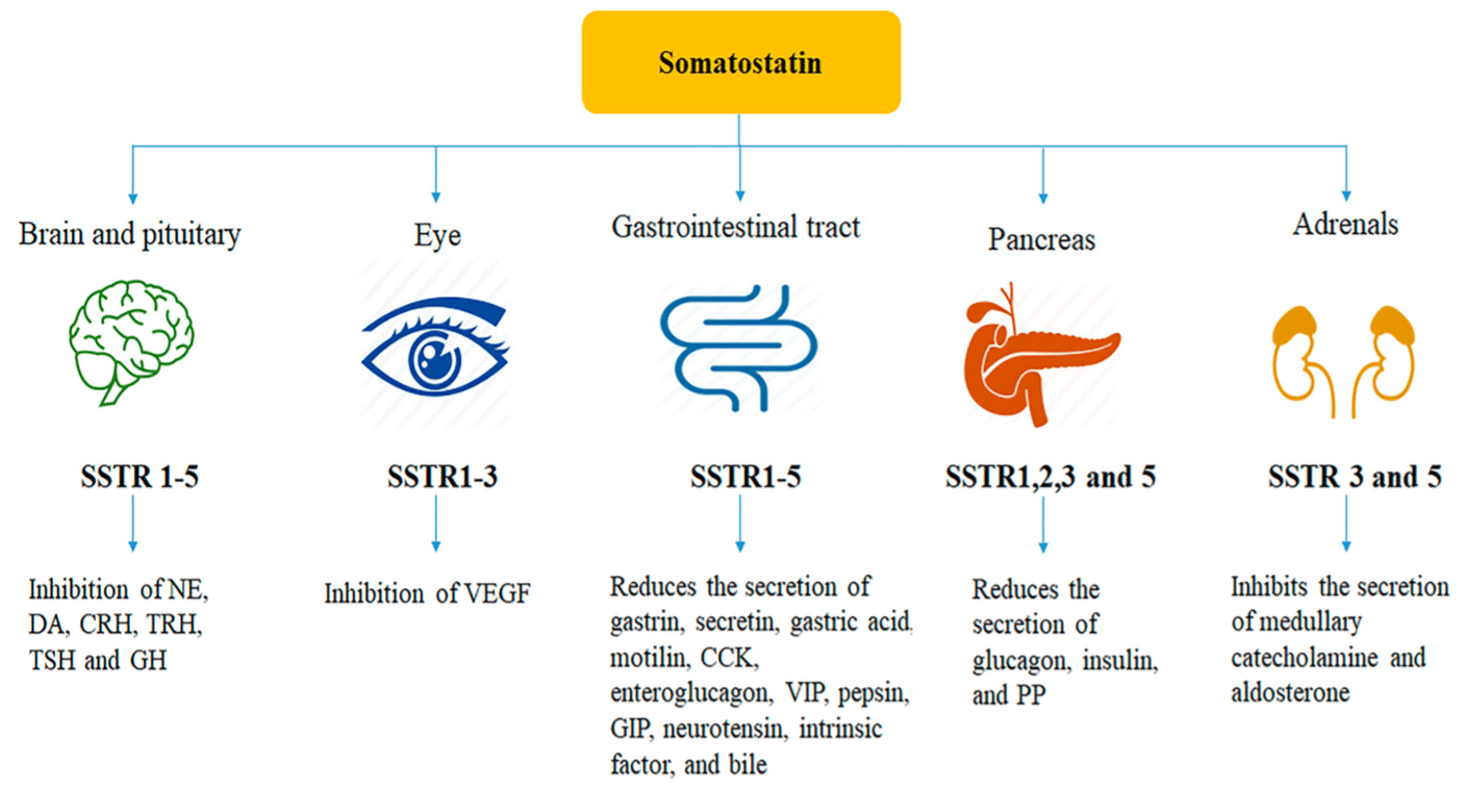
Somatostatin (SST), discovered in 1973, is a synthetic cyclic 14-amino acid tetradecapeptide hormone that exerts multiple biological activities via five ubiquitously distributed receptor subtypes.[i] The five receptor subtypes are SSTR1–SSTR5, with all five subtypes are expressed in normal human tissues. The predominant subtypes in endocrine tissues are SSTR2 and SSTR5, with neuroendocrine neoplasms have repeatedly been reported to express SSTR2 and SSTR5.[ii]
SST is considered a universal endocrine molecule and peptide hormone in the CNS, PNS and ENS.[iii] SST is classified as a broad inhibitory neuropeptide. SST inhibits the secretion of several other hormones, including growth hormone, gastrin, glucagon, thyroid stimulating hormone, cholecystokinin, secretin, insulin, pancreatic polypeptide, vasoactive intestinal peptide (VIP), 5-Hydroxytryptamine (5-HT) and some anterior pituitary hormones.[iv] Due to its effect on growth hormone, it is also referred to as growth hormone inhibiting hormone (GHIH).
SST also has anti-secretory, anti-proliferative and anti-angiogenic effects with glucose, fat, and amino acid absorption also being inhibited by STT. STT modulates gastrointestinal motility, delaying the late phase of gastric emptying, weakening gallbladder contraction, and prolonging small-intestinal transit time, but it also accelerates early gastric emptying and shortens the interval between migrating motor complexes.[v] SST decreases portal (and varicose) pressure, inhibits retinal secretion and has an antidiuretic effect in humans. It is also believed that SST modulates activities of the central nervous system that underlie cognition and locomotion.[vi] An inhibition of immunoglobulin synthesis and lymphocyte proliferation in lymphoid tissues has also been observed. An antiproliferative potential was also demonstrated by reversing the impact of mitogenic signals delivered by substances such as epidermal growth factor (EGF) and insulin-like growth factor 1 (IGF-1).[vii]
The clinical use of native somatostatin is limited by a very short half-life (1 – 3min) and the broad spectrum of biological responses.[viii] Consequently, stable, receptor-selective agonists have been developed, with most of these somatostatin therapeutic agonists bind strongly to two of the five receptor subtypes.
The reference range for plasma somatostatin in adults is 10-22 pg/ml.
Effects of Somatostatin – Gastrointestinal System
Production – SST is produced by in the D cells of the stomach and duodenum, and the δ cells of the islets of Langerhans of the pancreas. SST secretion is stimulated by the presence of glucose, amino acids and glucagon-like peptide-1. SST has numerous regulatory functions in the gastrointestinal system. Its primary role in gastric physiology is to inhibit both gastrin release and parietal cell acid secretion.
Effect on Gastric Acid Secretion
Somatostatin inhibits gastric acid secretion via an indirect and a direct pathway.
Direct Pathway
Depending on the source of the SS, the hormone can act in a paracrine or an endocrine fashion.
In the stomach, D cells are found near the base of the oxyntic glands, the predominant gland type within the body and fundus of the stomach. When released into the stomach, SS binds to an alpha-1 G-protein coupled receptor on the basolateral membrane of the parietal cell. This binding leads to the inhibition of adenylyl cyclase, antagonizing the stimulatory effect of histamine, and thus inhibiting gastric acid secretion by parietal cells.
Indirect Pathway
SS can activate two indirect paracrine pathways on G and D cells of the stomach. Histamine and gastrin are both examples of secretagogues which are substances that cause another substance to be secreted and due to this function, SS can inhibit gastric acid secretion. In the corpus of the stomach, D cells release the hormone to inhibit the release of histamine from the Enterochromaffin-like cells (ECL cells). In the antrum of the stomach, the release of SS from the D cells inhibits the release of gastrin from G cells. This is an example of redundant regulatory pathways that control acid secretion.
Effects in the Pancreas
In the pancreas, SS is secreted by δ-cells (delta cells). Once released, it acts as a powerful inhibitor of glucagon and insulin secretion from the α- and β-cells, respectively. Glucose stimulates SS secretion via G-proteins. When blood glucose concentrations are high, activation of cellular receptors causes the closure of ATP-sensitive K+ channels, initiating membrane depolarization and increasing SS release from delta cells. This is comparable to the mechanism of insulin secretion. It also suppresses pancreatic exocrine secretions through the inhibition of cholecystokinin-stimulated enzyme secretion and secretin-stimulated bicarbonate secretion.
Summary of GI Effects
- Decreased gastrinrelease leading to reduced gastric acid
- Increased fluid absorption
- Increased smooth muscle contraction
- Paracrine inhibition of insulin and glucagon secretion from α and β-cells of the Islets of Langerhans
- Decreased bile flow
- Decreased blood glucose concentration
Effects of Somatostatin – Nervous System
SST is produced in the brain as well as the gastrointestinal tract. A key producer of this neuropeptide is the hypothalamus, which predominantly synthesizes the 14 amino acid form of the hormone in the periventricular region. SS-14 is released into the hypophyseal portal blood system. Again, SST is a potent Growth Hormone (GH) inhibitor, thus is sometimes referred to as Growth Hormone Inhibiting Hormone (GHIH). Whilst many hypothalamic-pituitary axes are controlled through a negative feedback mechanism, control of GH is regulated through both positive and negative control.
Summary of Neurological Effects
- Inhibits secretion of growth hormone GH from the anterior pituitary
- Inhibits secretion of thyroid-stimulating hormone from the anterior pituitary
Synthetic Analogs
Somatostatin agonists, with longer half-lives than somatostatin, are established in the treatment of acromegaly with recently approved indications in the therapy of neuroendocrine tumors.[ix] After the discovery and characterization of SST, PHARMA developed SST synthetic analogs (SSAs) with longer half-life. To date, three of them have been approved in clinical practice: lanreotide (t1/2 90min and 4.5days for LR) and octreotide (t1/2 113min and LAR 1.7-1.9hr) are considered first-generation SSAs, and pasireotide (higher affinity for SSTR receptors) is considered a second-generation SSA. Their main clinical uses have been evaluated in Phase III clinical Trials, and some other uses have been evaluated in prospective studies.
Potential therapeutic uses for somatostatin analogues include diabetic complications like retinopathy, nephropathy, and obesity, due to inhibition of IGF-1, VEGF together with insulin secretion and effects upon the renin-angiotensin-aldosterone system.[x] Wider uses in anti-neoplastic therapy may also be considered and recent studies have further revealed anti-inflammatory and anti-nociceptive effects.
[i] Brazeau P., Vale W., Burgus R., Ling N., Butcher M., Rivier J., Guillemin R. Hypothalamic polypeptide that inhibits the secretion of immunoreactive pituitary growth hormone. Science. 1973;179:77–79.
[ii] Patel YC. Somatostatin and its receptor family. Front. Neuroendocrinol. 1999;20:157–198.
[iii] Gonkowski S, Rytel L. Somatostatin as an active substance in the mammalian enteric nervous system. Int J Mol Sci. 2019;20:4461.
[iv] Brazeau P, et al. Hypothalamic polypeptide that inhibits the secretion of immunoreactive pituitary growth hormone. Science. 1973;179:77-79.
[v] Shamsi BH, et al. Versatile Functions of Somatostatin and Somatostatin Receptors in the Gastrointestinal System. Front Endocrinol. 2021;12:652363.
[vi] Martel G., Dutar P., Epelbaum J., Viollet C. Somatostatinergic systems: An update on brain functions in normal and pathological aging. Front. Endocrinol. 2012;3:154.
[vii] Møller LN,e t al. Somatostatin receptors. Biochim. Biophys. Acta. 2003;1616:1–84.
[viii] Benuck M., Marks N. Differences in the degradation of hypothalamic releasing factors by rat and human serum. Life Sci. 1976;19:1271–1276.
[ix] Rai U, et al. Therapeutic uses of somatostatin and its analogues: Current view and potential applications. Pharmacol Ther. 2015;152:98-110.
[x] Gomes-Porras M, et al. Somatostatin analogs in clinical practice: a review. Int J Mol Sci. 2020;21(5):1682.
Clinical Research
IPS Level of Evidence
IPS Clinical Pharmacists have developed a method of ranking the studies so that the practitioner can easily discern the level of evidence this study provides to the topic. Levels 1-8 are listed below:
| Level of Evidence | Description | |
| X | Level 1 | FDA Approved Drug studies |
| Level 2 | Evidence obtained from systematic review and/or meta-analyses of studies including RCTs and other human studies | |
| X | Level 3 | Evidence obtained from a RCT |
| X | Level 4 | Evidence obtained from a study without randomization |
| Level 5 | Evidence obtained from case reports | |
| Level 6 | Evidence obtained from in vitro human studies | |
| X | Level 7 | Evidence obtained from laboratory animal studies |
| X | Level 8 | Evidence obtained from Opinions or Reviews |
Levels
Level 1
Melmed S, et al. Safety and efficacy of oral octreotide in acromegaly: results of a multicenter phase III trial. J Clin Endocrinol Metab. 2015;100(4):1699-708.
Abstract
Background: A novel oral octreotide formulation was tested for efficacy and safety in a phase III, multicenter, open-label, dose-titration, baseline-controlled study in patients with acromegaly.
Methods: We enrolled 155 complete or partially controlled patients (IGF-1 <1.3 × upper limit of normal [ULN], and 2-h integrated GH <2.5 ng/mL) receiving injectable somatostatin receptor ligand (SRL) for ≥ 3 months. Subjects were switched to 40 mg/d oral octreotide capsules (OOCs), and the dose escalated to 60 and then up to 80 mg/d to control IGF-1. Subsequent fixed doses were maintained for a 7-month core treatment, followed by a voluntary 6-month extension.
Results: Of 151 evaluable subjects initiating OOCs, 65% maintained response and achieved the primary endpoint of IGF-1 <1.3 × ULN and mean integrated GH <2.5 ng/mL at the end of the core treatment period and 62% at the end of treatment (up to 13 mo). The effect was durable, and 85 % of subjects initially controlled on OOCs maintained this response up to 13 months. When controlled on OOCs, GH levels were reduced compared to baseline, and acromegaly-related symptoms improved. Of 102 subjects completing the core treatment, 86% elected to enroll in the 6-month extension. Twenty-six subjects who were considered treatment failures (IGF-1 ≥ 1.3 × ULN) terminated early, and 23 withdrew for adverse events, consistent with those known for octreotide or disease related.
Conclusions: OOC, an oral therapeutic peptide, achieves efficacy in controlling IGF-1 and GH after switching from injectable SRLs for up to 13 months, with a safety profile consistent with approved SRLs. OOC appears to be effective and safe as an acromegaly monotherapy.
Level 3
Newman CB, et al. Ocreotide as primary therapy for acromegaly. J Clin Endocrinol Metab. 1998;83(9):3034-40.
Abstract
The effects of octreotide (up to 5 yr) as primary treatment in 26 patients with acromegaly were compared with those in 81 patients with acromegaly who received octreotide as secondary or adjunctive therapy after previous surgery and/or pituitary radiation. These patients were part of a multicenter study that took place between 1989-1995. The study was divided into 3 phases beginning with a 1-month placebo-controlled treatment period followed by a 1-month washout period. In the second phase, patients were randomized to treatment with either 100 or 250 micrograms octreotide, sc, every 8 h for 6 months. Octreotide was then discontinued for 1 month and reinitiated at the lower dose for a total mean treatment duration of 39 months. The dose was titrated by each investigator to improve each patient’s individual response, which included improvement in symptoms and signs of acromegaly as well as reduction of GH and insulin-like growth factor I (IGF-I) into the normal range. In the second phase of the study, in which patients were randomized to either 100 or 250 micrograms octreotide, three times daily, mean integrated GH and IGF-I concentrations after 3 and 6 months were equivalent in the primary and secondary treatment groups. During long term open label treatment, mean GH fell from 32.7 +/- 5.2 to 6.0 +/- 1.7 micrograms/L 2 h after octreotide injection in the primary therapy group and remained suppressed for a mean period of 24 months (range, 3-60 months). The mean final daily dose was 777 micrograms. In the patients receiving secondary treatment, mean GH fell from 30.2 +/- 7.6 to 5.6 +/- 1.1 micrograms/L after 3 months and remained suppressed for the remainder of the study (average dose, 635 micrograms daily). Mean IGF-I concentrations fell from 5.2 +/- 0.5 x 10(3) U/L (primary treatment group) and 4.7 +/- 0.4 x 10(3) U/L (secondary treatment group) to a mean of 2.2 +/- 0.3 x 10(3) U/L in both groups after 3 months of open label treatment and remained suppressed. IGF-I was reduced into the normal range during at least half of the study visits in 68% of the primary treatment group and in 62% of the secondary treatment group. Patients whose GH levels fell to at least 2 SD below the baseline mean GH were considered responders. There was no significant difference in the percentage of responders in the primary and secondary treatment groups (70% vs. 61%), nor was there a statistical difference in the mean GH concentrations between the groups. Symptoms of headache, increased perspiration, fatigue, and joint pain were reported at baseline by 46%, 73%, 69%, and 85%, respectively, of patients in the primary therapy group and improved during 3 yr of octreotide treatment in 50-100%. Similarly, these acromegaly-related symptoms were reported by 62%, 58%, 78%, and 60% of patients in the secondary therapy group, and improvement was noted in 62-88%. Pituitary magnetic resonance imaging scans were available in 13 of 26 patients in the primary treatment group before and after 6 months of octreotide treatment. Tumor shrinkage was observed in 6 of 13 patients, with reduction in tumor volume greater than 25% in only 3. Of 6 patients with documented tumor shrinkage, IGF-I was reduced into the normal range in 4 patients. Of the 7 remaining patients in whom tumor shrinkage was less than 10%, IGF-I was reduced into the normal range in 4 patients. Of the 7 remaining patients in whom tumor shrinkage was less than 10%, IGF-I was reduced into the normal range in 5 patients. The degree of tumor shrinkage did not correlate with the percent reduction in IGF-I or GH. In summary, octreotide was equally effective in 26 previously untreated acromegalic patients (primary treatment group) and 81 patients previously treated with either surgery or pituitary radiation (secondary treatment group). These observations call into question the current practice of surgical resection of all newly diagnosed GH-secreting pituitary adenomas regardless of the likelihood of cure.
https://sci-hub.se/10.1210/jcem.83.9.5109
Prencipe N, et al. Biliary adverse events in acromegaly during somatostatin receptor ligands: predictors of onset and response to ursodeoxycholic acid treatment. Pituitary. 2021;24(2):242-51.
Abstract
Purpose; Somatostatin receptor ligands (SRL) are the first-line medical treatment for acromegaly. Gallbladder alterations are one of most important SRL side effect, but according to some authors growth hormone hypersecretion itself is a risk factor for gallstones. This single center, longitudinal retrospective study evaluated the incidence and the predictors of biliary adverse events (BAE) in acromegaly during SRL therapy and their response to ursodeoxycholic acid (UDCA).
Methods: 91 acromegaly patients with indication to SRL were enrolled. Evaluations of acromegaly activity (GH, IGF-I, IGF-I/ULN) and metabolic profile were collected before starting treatment, yearly during follow-up and at BAE onset. In patients developing BAE we searched for predictors of UDCA effectiveness.
Results: 61.5% of patients developed BAE (58.9% cholelithiasis; 41.1% only sludge). IGF-I and IGF-I/ULN proved to be positive predictor of BAE, which occur about 5 years after SRL starting. None of metabolic markers proved to be associated with BAE. Only five patients (5.5%) underwent cholecystectomy for symptomatic cholelithiasis. 71% of patients started UDCA treatment, achieving regression of BAE in 60% of cases (88% in patients developing only sludge and 30% in patients affected by cholelithiasis, p < 0.001). BMI and obesity were negative predictors of UDCA efficacy. In 50% of the subjects BAE resolved after 36 months of therapy with a lower rate if cholelithiasis was present.
Conclusion: Biliary stone disease is a frequent SRL adverse event, although it is often symptomless. Ultrasound follow-up mainly in the first 5 years of therapy, early UDCA starting and proper lifestyle represent a valid strategy in their detection and management.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7966199/
Newman CB, et al. Safety and efficacy of long-term octreotide therapy of acromegaly: results of a multicenter trial in 103 patients–a clinical research center study. J Clin Endocrinol Metab. 1995;80(9):2768-75.
Abstract
One hundred and three acromegalic patients from 14 medical centers were enrolled in this study to determine the efficacy and safety of the somatostatin analog, octreotide acetate, during long term treatment. Seventy percent of the patients had undergone previous surgery or radiation treatment. Octreotide was initiated at a dose of 100 micrograms, sc, every 8 h and gradually increased to a maximum of 1500 micrograms daily depending upon the individual patient’s clinical and biochemical response [GH and insulin-like growth factor I (IGF-I) reduction]. The mean duration of treatment was 24 months (range, 3-30 months). However, most patients were treated for a mean of 30 months, because this study took place after an initial 6-month study previously reported. Mean serum GH fell from 30.9 micrograms/L (range, 2.7-350) to 5.7 micrograms/L (range, 0.6-59) at the 3 months visit and remained suppressed (P < 0.001). Plasma IGF-I concentrations were also significantly reduced and remained in the normal range for at least half of the treatment visits in 56 of 87 patients (64%) treated for 12-30 months. Patients with higher initial GH concentrations were less likely to normalize IGF-I concentrations during treatment (P < 0.001). There was no evidence of drug tachyphylaxis in those patients who continued taking stable doses of medication. With some exceptions, dose increments above 800 micrograms daily in 31 patients did not provide additional benefit in terms of GH and IGF-I reduction. Headache, excessive perspiration, fatigue, and joint pain were ameliorated in 83-95% of patients. Mean finger circumference was decreased significantly at the 12 month visit (P < 0.05). The most common adverse events reported were diarrhea, abdominal discomfort, loose stools, and nausea; these symptoms usually disappeared within 3 months of treatment. Five patients discontinued octreotide because of adverse events. Of 102 patients with normal baseline ultrasound examinations of the gallbladder, 24 patients (23.5%) developed gallstones (usually during the first year of treatment), and 21 patients developed sludge alone. Gallstone formation was not related to the dose of octreotide. Most patients with cholelithiasis were asymptomatic, and none developed cholecystitis. These observations suggest that octreotide is a valuable long term medical treatment for acromegaly.
Level 4
Lamberti G, et al. Nonconventional Doses of Somatostatin Analogs in Patients With Progressing Well-Differentiated Neuroendocrine Tumor. J Clin Endocrinol Metab. 2019;
Abstract
Purpose: To evaluate the antiproliferative activity and safety of nonconventional high doses of somatostatin analogs (HD-SSA) in patients with well-differentiated gastroenteropancreatic (GEP) neuroendocrine tumors (NET) with radiological disease progression according to Response Evaluation Criteria in Solid Tumors (RECIST) criteria on a previous treatment.
Methods: A retrospective analysis of prospectively maintained databases from 13 Italian NET-dedicated centers was performed. Main inclusion criteria were: well-differentiated G1 or G2 GEP-NET, progressive disease on a previous treatment, and subsequent treatment with HD-SSA (either by increased administered dose [dose intensity] or shortened interval between administrations [dose density]). Main endpoints were progression-free survival (PFS) and safety.
Results: Of 198 patients, 140 matched inclusion criteria and were included in the analysis. Overall, median PFS was 31 months. Use of HD-SSA as second-line treatment was associated with reduced risk for progression or death compared with third- or further-line treatment (HR: 2.12; P = 0.004). There was no difference in PFS between HD-SSA by increased dose density (N = 133; 95%) or intensity (N = 7; 5%). Partial response according to RECIST criteria was observed in 12 patients (8.6%), and stable disease was achieved in 106 (75.7%) patients. Adverse events occurred in 21 patients (15.0%), 2 of whom had grade 3 biliary stone disease. No patients discontinued HD-SSA treatment due to adverse events.
Conclusions: HD-SSA is an active and safe treatment option in patients with progressive well-differentiated GEP-NET. The high rate of objective responses observed deserves prospective validation in ad hoc clinical trials.
Level 7
Brazeau P, et al. Hypothalamic polypeptide that inhibits the secretion of immunoreactive pituitary growth hormone. Science. 1973;179:77-79.
Abstract
A peptide has been isolated from ovine hypothalamus which, at 1 x 10(-9)M, inhibits secretion in vitro of immunoreactive rat or human growth hormones and is similarly active in vivo in rats. Its structure is H-Ala-Gly-Cys-Lys-Asn-Phe-Phe-Trp-Lys-Thr-Phe-Thr-Ser-Cys-OH The synthetic replicate is biologically active.
Level 8
Gadelha M, et al. The future of somatostatin receptor ligands in acromegaly. J Clin Endocrinol Metab. 2022;107(2):297-308.
Abstract
Currently, the first-generation somatostatin receptor ligands (fg-SRLs), octreotide LAR and lanreotide autogel, are the mainstays of acromegaly treatment and achieve biochemical control in approximately 40% of patients and tumor shrinkage in over 60% of patients. Pasireotide, a second-generation SRL, shows higher efficacy with respect to both biochemical control and tumor shrinkage but has a worse safety profile. In this review, we discuss the future perspectives of currently available SRLs, focusing on the use of biomarkers of response and precision medicine, new formulations of these SRLs and new drugs, which are under development. Precision medicine, which is based on biomarkers of response to treatment, will help guide the decision-making process by allowing physicians to choose the appropriate drug for each patient and improving response rates. New formulations of available SRLs, such as oral, subcutaneous depot, and nasal octreotide, may improve patients’ adherence to treatment and quality of life since there will be more options available that better suit each patient. Finally, new drugs, such as paltusotine, somatropin, ONO-5788, and ONO-ST-468, may improve treatment adherence and present higher efficacy than currently available drugs.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8764337/
Delia D, et al. Patient and Healthcare Provider Perspectives of First-Generation Somatostatin Analogs in the Management of Neuroendocrine Tumors and Acromegaly: A Systematic Literature Review. Adv Ther. 2021;38(2):969-993.
Abstract
IntroductionSomatostatin analogs (SSAs) are used to treat neuroendocrine tumors (NETs) and acromegaly. Two first-generation SSAs, octreotide long-acting release (OCT LAR) and lanreotide autogel/depot (LAN), are available. A systematic literature review (SLR) was conducted to investigate which characteristics beyond efficacy are most important in patient and healthcare practitioner (HCP) experience of LAN and OCT when used to treat acromegaly and NETs.
Methods: MEDLINE, Embase, the Cochrane Library, and Database of Abstracts of Reviews of Effect were searched from database inception to January 2019 with terms for first-generation SSAs, NETs, acromegaly, preferences, decision-making, and human factors. Key congresses in 2016–2018 and SLR bibliographies were hand-searched. Two independent reviewers screened articles at title/abstract and full-text stage. Publications fulfilling pre-specified inclusion criteria reported patient or HCP perspectives of LAN or OCT, or any factors affecting treatment perspectives for NETs or acromegaly.
Results: A total of 1110 unique records were screened, of which 21 studies were included, reporting from the perspectives of patients (n = 18) and/or HCPs (n = 9). Perspectives were collected using shared decision-making frameworks, questionnaires, informal patient opinion, and a Delphi panel. Where patient preference was specifically reported, LAN was preferred in 4/5 studies and OCT LAR in 1/5. Common factors underlying treatment experience included technical problems with injections and associated pain, emotional quality/anxiety of injections, time and convenience of treatment administration, and independence. Immediate aspects of injections appeared most important to patients, though the possibilities of extended dosing intervals and self-/partner-injection with LAN were also notable factors.
Conclusions: Study outcomes favored LAN in this SLR, with factors surrounding injection administration most influential in treatment experience. The findings of this SLR provide a basis that could inform development of decision-making criteria, with patient and HCP treatment perspectives considered. Future studies should utilize a common method to report preference and associated drivers.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7799425/pdf/12325_2020_Article_1600.pdf
Li J, et al. Somatostatin and Octreotide on the treatment of acute pancreatitis – basic and clinical studies for 3 decades. Curr Pharm Design. 2011;17:1594-1601.
Abstract
The finding that somatostatin (SST) or its analogue, octreotide caused a dose-dependent reduction in exocrine pancreatic secretions triggered their research as therapeutic options for acute pancreatitis (AP), a life-threatening illness. However, the accumulative clinical trials of SST or octreotide in AP treatment present the controversial results. The insufficient secretory capacity of acinar cells in AP patients also queries the validity of traditional assumption on the effects of SST and octreotide. This reviewer updates the current pharmacological concepts of SST and octreotide in treatment of AP and the clinical strategies of their application. The impressive inhibitive effects on the massive inflammatory injury via the several signal pathways make SST as an important anti-inflammatory peptide. Furthermore, endocrine SST may decrease the sphincter tone of Oddi (SO) by its potential role on the neurocrine of SST in SO. Therefore, it should be rational that exogenous supplement of SST or octreotide for AP patients due to the plasma level of SST during AP is usually lower than that of normal. In accordance with these findings, the optimal stage of SST or octreotide administration, application in high-risk population, the cost-effective dosage or duration as well as the design of suitable clinical outcomes would be the interesting topics of translational medical research for both basic scientists and clinicians.
https://sci-hub.se/10.2174/138161211796196936
Rogoza O, et al. Role of somatostatin signaling in neuroendocrine tumors. Int J Mol Sci. 2022;23(3):1447.
Abstract
The finding that somatostatin (SST) or its analogue, octreotide caused a dose-dependent reduction in exocrine pancreatic secretions triggered their research as therapeutic options for acute pancreatitis (AP), a life-threatening illness. However, the accumulative clinical trials of SST or octreotide in AP treatment present the controversial results. The insufficient secretory capacity of acinar cells in AP patients also queries the validity of traditional assumption on the effects of SST and octreotide. This reviewer updates the current pharmacological concepts of SST and octreotide in treatment of AP and the clinical strategies of their application. The impressive inhibitive effects on the massive inflammatory injury via the several signal pathways make SST as an important anti-inflammatory peptide. Furthermore, endocrine SST may decrease the sphincter tone of Oddi (SO) by its potential role on the neurocrine of SST in SO. Therefore, it should be rational that exogenous supplement of SST or octreotide for AP patients due to the plasma level of SST during AP is usually lower than that of normal. In accordance with these findings, the optimal stage of SST or octreotide administration, application in high-risk population, the cost-effective dosage or duration as well as the design of suitable clinical outcomes would be the interesting topics of translational medical research for both basic scientists and clinicians.
https://sci-hub.se/10.2174/138161211796196936
La Salvia A, et al. Somatostatin Analogue Therapy in MEN1-Related Pancreatic Neuroendocrine Tumors from Evidence to Clinical Practice: A Systematic Review. Pharmaceuticals (Basel). 2021;14(10):1039.
Abstract
Neuroendocrine neoplasms (NENs) are relatively rare and complex tumors that can be sporadic or hereditary, as in the context of multiple endocrine neoplasia type 1 (MEN1) where patients display a 70% lifelong risk of developing a pancreatic NENs (pNENs). To date, specific personalized treatment for pNENs in patients with MEN1 are lacking. The aim of this study was to systematically analyze the efficacy and safety of somatostatin analogue (SSA) treatment in patients affected by MEN1-related pNENs. We performed a systematic review of the literature, searching for peer-reviewed articles on SSA (octreotide or lanreotide) treatment in MEN1 associated with pNENs. We selected 20 studies with a pooled population of 105 MEN1 patients with pNENs. Females were 58.5%, median age was 44 years (18-73). TNM stage at diagnosis was stage I-II in 84.8% and stage IV in 15.2%. The overall response rate (SD+PR+CR) was achieved in 88.3% of cases, with stable disease in 75.6% and objective response in 12.7% of patients. The safety profile was favorable with both SSA agents. SSAs appear to be an effective and safe treatment option for MEN1-related pNEN, either at localized or advanced stages.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8538402/
Gomes-Porras M, et al. Somatostatin analogs in clinical practice: a review. Int J Mol Sci. 2020;21(5):1682.
Abstract
Somatostatin analogs are an invaluable therapeutic option in the diagnosis and treatment of somatotropinomas, thyrotropinomas, and functioning and non-functioning gastroenteropancreatic neuroendocrine tumors. They should also be considered an effective and safe therapeutic alternative to corticotropinomas, gonadotropinomas, and prolactinomas resistant to dopamine agonists. Somatostatin analogs have also shown to be useful in the treatment of other endocrine diseases (congenital hyperinsulinism, Graves’ orbitopathy, diabetic retinopathy, diabetic macular edema), non-endocrine tumors (breast, colon, prostate, lung, and hepatocellular), and digestive diseases (chronic refractory diarrhea, hepatorenal polycystosis, gastrointestinal hemorrhage, dumping syndrome, and intestinal fistula).
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7084228/pdf/ijms-21-01682.pdf
Rai U, et al. Therapeutic uses of somatostatin and its analogues: Current view and potential applications. Pharmacol Ther. 2015;152:98-110.
Abstract
Somatostatin is an endogeneous cyclic tetradecapeptide hormone that exerts multiple biological activities via five ubiquitously distributed receptor subtypes. Classified as a broad inhibitory neuropeptide, somatostatin has anti-secretory, anti-proliferative and anti-angiogenic effects. The clinical use of native somatostatin is limited by a very short half-life (1 to 3 minutes) and the broad spectrum of biological responses. Thus stable, receptor-selective agonists have been developed. The majority of these somatostatin therapeutic agonists bind strongly to two of the five receptor subtypes, although recently an agonist of wider affinity has been introduced. Somatostatin agonists are established in the treatment of acromegaly with recently approved indications in the therapy of neuroendocrine tumors. Potential therapeutic uses for somatostatin analogues include diabetic complications like retinopathy, nephropathy and obesity, due to inhibition of IGF-1, VEGF together with insulin secretion and effects upon the reninangiotensin-aldosterone system. Wider uses in anti-neoplastic therapy may also be considered and recent studies have further revealed anti-inflammatory and anti-nociceptive effects. This review provides a comprehensive, current view of the biological functions of somatostatin and potential therapeutic uses, informed by the wide range of pharmacological advances reported since the last published review in 2004 by P. Dasgupta. The pharmacology of somatostatin receptors is explained, the current uses of somatostatin agonists are discussed, and the potential future of therapeutic applications is explored.
https://sci-hub.se/10.1016/j.pharmthera.2015.05.007
Gillis JC, et al. Octreotide long-acting release (LAR). A review of its pharmacological properties and therapeutic use in the management of acromegaly. Drugs. 1997;43(4):681-99.
Abstract
Octreotide is a somatostatin analogue: a long-acting release (LAR) formulation of octreotide is designed for once-monthly intramuscular administration. As with native somatostatin, octreotide LAR exerts potent inhibitory effects on the secretion of growth hormone and on various peptides of the gastroenteropancreatic endocrine system. When patients with acromegaly who show a positive response to treatment with subcutaneous octreotide 300 to 600 micrograms/day are switched to octreotide LAR 20 or 30 mg, the resulting decrease in growth hormone levels is stable and sustained. Reductions in growth hormone levels to < 5 micrograms/L for about 4 weeks are seen in 86 to 100% of patients, to < 2 to 2.5 micrograms/L in 39 to 75% and to < 1 microgram/L in 24 to 40%. Levels of insulin-like growth factor-1 (IGF-1) decrease in parallel and are often normalised with repeated drug treatment. There is no evidence of tachyphylaxis with long term therapy (up to 34 months). Treatment with octreotide LAR improves facial appearance and soft tissue thickening, and eliminates or reduces the incidence of symptoms such as headache, fatigue, arthralgia and excessive perspiration. Tumour shrinkage has been noted in some, but not all, patients receiving octreotide LAR, although this has not been widely evaluated in clinical studies. Overall, octreotide LAR is well tolerated, and the mild to moderate gastrointestinal events experienced by up to 50% of patients are of short duration and often subside with continued drug administration. The incidence of gallbladder abnormalities (sediment, sludge, microlithiasis and gallstones) increases in patients receiving long term therapy with subcutaneous octreotide, although most patients remain asymptomatic. The incidence of gallbladder abnormalities in patients receiving octreotide LAR compares favourably with that during subcutaneous administration. Glycaemic control is not usually altered during octreotide LAR treatment. In summary, octreotide continues to be the principal pharmacological option for most patients with acromegaly. Octreotide LAR offers the convenience of once-monthly administration compared with daily subcutaneous drug administration. In addition, the good efficacy and tolerability profile of octreotide LAR should enhance patient compliance and acceptability of octreotide therapy and contribute to an improvement in patient quality of life.
No Full Text Available
Lowell A. From somatostatin to octreotide LAR: evolution of a somatostatin analogue. Curr Med Res Opin. 2009;25(12):2989-2999.
Abstract
Background: Acromegaly is characterized by overproduction of growth hormone (GH) by the pituitary gland. GH stimulates the synthesis of insulin-like growth factor-I (IGF-I), and the somatic growth and metabolic dysfunction that characterize acromegaly are a consequence of elevated GH and IGF-I levels. Gastroenteropancreatic neuroendocrine tumors (GEP-NETs) are rare, slow-growing neoplasms that have usually metastasized by the time of diagnosis. The majority of GEP-NETs are carcinoid tumors whose syndrome is caused by the hypersecretion of biogenic amines, peptides and polypeptides responsible for the principal symptoms of diarrhea and flushing.
Methods: The MEDLINE and EMBASE databases were searched for preclinical and clinical studies of octreotide (Sandostatin*), a potent synthetic somatostatin analogue, in patients with acromegaly or GEP-NETs. Objective: This article reviews the 20 years of clinical experience with octreotide and the impact it has made in patients with acromegaly or GEP-NETs.
Results: Octreotide has proven to be an essential component in the management strategy of acromegaly and GEP-NETs over the past 20 years. The multiple beneficial effects of octreotide throughout the body, combined with its established safety profile (the most common adverse effects are injection-site pain and gastrointestinal events), have made it an appealing option for clinicians. The advent of the long-acting release (LAR) formulation of octreotide provided additional benefits to patients through monthly administration, while maintaining the efficacy and tolerability profile of the daily subcutaneous formulation.
Conclusions: Octreotide is a potent synthetic somatostatin analogue that has become the mainstay of medical therapy for tumor control in neuroendocrine disorders such as acromegaly and GEP-NETs. The development of octreotide LAR offered a further advancement; less frequent dosing provided valuable benefits in quality of life to patients, with equivalent efficacy and tolerability. Moreover, recent results from the PROMID study have confirmed the antiproliferative effect of octreotide LAR in patients with well-differentiated metastatic GEP-NETs of the midgut. New therapeutic uses of octreotide are currently under investigation in a variety of clinical settings.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3678951/pdf/nihms-475890.pdf
Patel YC, et al. Somatostatin and its receptor family. Front Neuroendocrinol. 1999;20(3):157-98.
Abstract
Somatostatin (SST), a regulatory peptide, is produced by neuroendocrine, inflammatory, and immune cells in response to ions, nutrients, neuropeptides, neurotransmitters, thyroid and steroid hormones, growth factors, and cytokines. The peptide is released in large amounts from storage pools of secretory cells, or in small amounts from activated immune and inflammatory cells, and acts as an endogenous inhibitory regulator of the secretory and proliferative responses of target cells that are widely distributed in the brain and periphery. These actions are mediated by a family of seven transmembrane ™ domain G-protein-coupled receptors that comprise five distinct subtypes (termed SSTR1-5) that are endoded by separate genes segregated on different chromosomes. The five receptor subtypes bind the natural SST peptides, SST-14 and SST-28, with low nanomolar affinity. Short synthetic octapeptide and hexapeptide analogs bind well to only three of the subtypes, 2, 3, and 5. Selective nonpeptide agonists with nanomolar affinity have been developed for four of the subtypes (SSTR1, 2, 3, and 4) and putative peptide antagonists for SSTR2 and SSTR5 have been identified. The ligand binding domain for SST ligands is made up of residues in TMs III-VII with a potential contribution by the second extracellular loop. SSTRs are widely expressed in many tissues, frequently as multiple subtypes that coexist in the same cell. The five receptors share common signaling pathways such as the inhibition of adenylyl cyclase, activation of phosphotyrosine phosphatase (PTP), and modulation of mitogen-activated protein kinase (MAPK) through G-protein-dependent mechanisms. Some of the subtypes are also coupled to inward rectifying K(+) channels (SSTR2, 3, 4, 5), to voltage-dependent Ca(2+) channels (SSTR1, 2), a Na(+)/H(+) exchanger (SSTR1), AMPA/ainite glutamate channels (SSTR1, 2), phospholipase C (SSTR2, 5), and phospholipase A(2) (SSTR4). SSTRs block cell secretion by inhibiting intracellular cAMP and Ca(2+) and by a receptor-linked distal effect on exocytosis. Four of the receptors (SSTR1, 2, 4, and 5) induce cell cycle arrest via PTP-dependent modulation of MAPK, associated with induction of the retinoblastoma tumor suppressor protein and p21. In contrast, SSTR3 uniquely triggers PTP-dependent apoptosis accompanied by activation of p53 and the pro-apoptotic protein Bax. SSTR1, 2, 3, and 5 display acute desensitization of adenylyl cyclase coupling. Four of the subtypes (SSTR2, 3, 4, and 5) undergo rapid agonist-dependent endocytosis. SSTR1 fails to be internalized but is instead upregulated at the membrane in response to continued agonist exposure. Among the wide spectrum of SST effects, several biological responses have been identified that display absolute or relative subtype selectivity. These include GH secretion (SSTR2 and 5), insulin secretion (SSTR5), glucagon secretion (SSTR2), and immune responses (SSTR2).
No Full Text Available
Shamsi BH, et al. Versatile Functions of Somatostatin and Somatostatin Receptors in the Gastrointestinal System. Front Endocrinol. 2021;12:652363.
Abstract
Somatostatin (SST) and somatostatin receptors (SSTRs) play an important role in the brain and gastrointestinal (GI) system. SST is produced in various organs and cells, and the inhibitory function of somatostatin-containing cells is involved in a range of physiological functions and pathological modifications. The GI system is the largest endocrine organ for digestion and absorption, SST-endocrine cells and neurons in the GI system are a critical effecter to maintain homeostasis via SSTRs 1-5 and co-receptors, while SST-SSTRs are involved in chemo-sensory, mucus, and hormone secretion, motility, inflammation response, itch, and pain via the autocrine, paracrine, endocrine, and exoendocrine pathways. It is also a power inhibitor for tumor cell proliferation, severe inflammation, and post-operation complications, and is a first-line anti-cancer drug in clinical practice. This mini review focuses on the current function of producing SST endocrine cells and local neurons SST-SSTRs in the GI system, discusses new development prognostic markers, phosphate-specific antibodies, and molecular imaging emerging in diagnostics and therapy, and summarizes the mechanism of the SST family in basic research and clinical practice. Understanding of endocrines and neuroendocrines in SST-SSTRs in GI will provide an insight into advanced medicine in basic and clinical research.
https://www.frontiersin.org/articles/10.3389/fendo.2021.652363/full
[1] Borna RM, Jahr JS, Kmiecik S, Mancuso KF, Kaye AD. Pharmacology of Octreotide: Clinical Implications for Anesthesiologists and Associated Risks. Anesthesiol Clin. 2017 Jun;35(2):327-339.
[2] Prencipe N, et al. Biliary adverse events in acromegaly during somatostatin receptor ligands: predictors of onset and response to ursodeoxycholic acid treatment. Pituitary. 2021;24(2):242-51.
[3] Brazeau P., Vale W., Burgus R., Ling N., Butcher M., Rivier J., Guillemin R. Hypothalamic polypeptide that inhibits the secretion of immunoreactive pituitary growth hormone. Science. 1973;179:77–79.
[4] Patel YC. Somatostatin and its receptor family. Front. Neuroendocrinol. 1999;20:157–198.
[5] Gonkowski S, Rytel L. Somatostatin as an active substance in the mammalian enteric nervous system. Int J Mol Sci. 2019;20:4461.
[6] Brazeau P, et al. Hypothalamic polypeptide that inhibits the secretion of immunoreactive pituitary growth hormone. Science. 1973;179:77-79.
[7] Shamsi BH, et al. Versatile Functions of Somatostatin and Somatostatin Receptors in the Gastrointestinal System. Front Endocrinol. 2021;12:652363.
[8] Martel G., Dutar P., Epelbaum J., Viollet C. Somatostatinergic systems: An update on brain functions in normal and pathological aging. Front. Endocrinol. 2012;3:154.
[9] Møller LN,e t al. Somatostatin receptors. Biochim. Biophys. Acta. 2003;1616:1–84.
[10] Benuck M., Marks N. Differences in the degradation of hypothalamic releasing factors by rat and human serum. Life Sci. 1976;19:1271–1276.
[11] Rai U, et al. Therapeutic uses of somatostatin and its analogues: Current view and potential applications. Pharmacol Ther. 2015;152:98-110.
[12] Gomes-Porras M, et al. Somatostatin analogs in clinical practice: a review. Int J Mol Sci. 2020;21(5):1682.