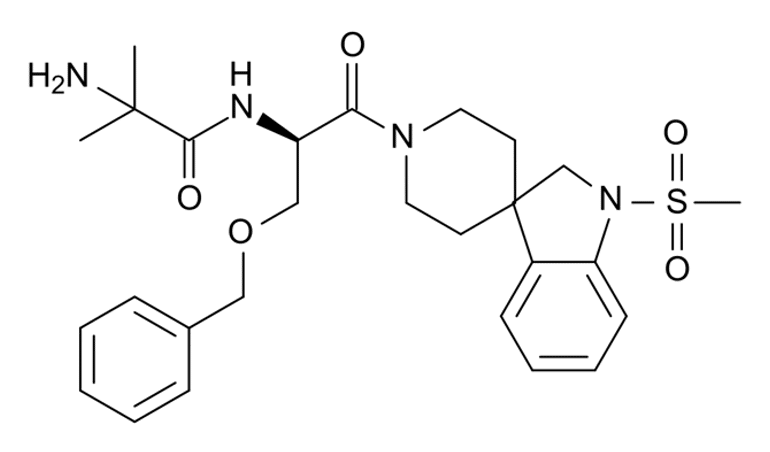
MK-677
IBUTAMOREN
MK-0677
GH SECRETAGOGUE (non-peptide)
GHRELIN MIMETIC
Contents
Most Frequent Uses:
- Long-acting orally active growth hormone secretagogue and ghrelin hormone mimetic
- Growth Hormone support
- Body composition improvement – weight loss, improved lean body mass
- Improved Performance
Dosage:
- General Dosage
- Orally, 10-25mg daily
Safety and Potential Side Effects/Contraindications:
- MK-677 administered orally is reported safe in recommended dosages.
- MK-677 may increase serum cortisol (lab and human studies). However, there are several human studies in humans using MK-677 where cortisol was not affected significantly.[i],[ii] Caution may be prudent in those with anxiety or other disorders where increased cortisol may be an issue.
[i] Murphy MG, et al. MK-677 an orally active growth hormone secretagogue, reverses diet-induced catabolism. J Clin Endocrinol.1998;83(2):320-25.
[ii] Svensson J, et al. Two-Month Treatment of Obese Subjects with the Oral Growth Hormone (GH) Secretagogue MK-677 Increases GH Secretion, Fat-Free Mass, and Energy Expenditure. J Clin Endocrinol Metabl. 1998;83(2):362-68.
Description
MK-677 is a nonpeptide, spiropiperidine sulfonamide reported functionally indistinguishable in vitro and in vivo from the peptide GH secretagogue GHRP-6.[i] MK-677 also activates ghrelin receptor. MK-677 is active after oral administration in animals and humans.[ii] It is reported to increase the secretion of GH, insulin-like growth factor 1 (IGF-1) and IGFBP-3 levels in children with GH deficiency. It also to produce sustained increases in the plasma levels of these hormones without affecting glucose, prolactin, triiodothyronine (T3), thyroxine (T4), insulin levels.[iii] Most studies report no effects on cortisol, but there are human and laboratory animal studies that report MK-677 increases cortisol.[iv]
MK-677, by sustaining activation of GH-IGF-1 axis, is reported in human studies to increase lean body mass with no change in total fat mass or visceral fat, and increases muscle mass and bone mineral density.[v],[vi] As such MK-677 is under investigation as a potential treatment for reduced levels of GH and IGF-1, such as in children with growth hormone deficiency or elderly with frailty issues.[vii],[viii],[ix] Patient benefits of MK-677 therapy over time may include: [x]
- Anti-aging
- Increase muscle mass
- Decrease fat mass
- Cholesterol improvement
- Faster healing
- Firmer healthier skin
- Healthier hair
- Improved sleep quality
MK-677 (ibutamoren) is still an Investigational New Drug in the US. MK-677 is on the WADA prohibited list and banned in sports.
[i] Patchett AA, et al. Design and biological activities of L-163,191 (MK-0677): a potent, orally active growth hormone secretagogue. Proc Natl Acad Sci USA. 1995;92:7001-5.
[ii] Patchett AA, et al. Design and biological activities of L-163,191 (MK-0677): a potent, orally active growth hormone secretagogue. Proc Natl Acad Sci USA. 1995;92:7001-5.
[iii] Copinschi G, et al. Effects of a 7 day treatment with a novel, orally active growth hormone secretagogue, MK-677 on 24 hr GH profiles insulin like growth factor1 and adrenocortical function in normal young men. The Journal of Clinical Endocrinology and Metabolism. 81 (8): 2776–82.
[iv] Naas R, et la. Effects of oral ghrelin mimetic on body composition and clinical outcomes in healthy older adults: a randomized, controlled trial. Ann Intern Med. 2008;149(9):601-11.
[v] Murphy MG, et al. Oral administration of the growth hormone secretagogue MK-677 increases markers of bone turnover in healthy and functionally impaired elderly adults. The MK-677 Study Group. J Bone Miner Res. 1999;14(7):1182-8.
[vi] Murphy MG, et al. Effect of alendronate and MK-677 (a growth hormone secretagogue), individually and in combination, on markers of bone turnover and bone mineral density in postmenopausal osteoporotic women. J clin Endocrinol Metab. 2001;86(3):1116-25.
[vii] Ibutamoren – Lumos Pharma/Merck – AdisInsight”.
[viii] Adunsky A, et al. MK-0677 (ibutamoren mesylate) for the treatment of patients recovering from hip fracture: a multicenter, randomized, placebo-controlled phase Iib study. Arch Gerontol Geriatr. 2011;53(2):183-9.
[ix] Svensson J, et al. Treatment with the oral growth hormone secretagogue MK-677 increases markers of bone formation and bone resorption in obese young males. J Bone Miner Res. 1998;13(7):1158-66.
[x] Codner E, et al. Effects of oral administration of ibutamoren mesylate, a nonpeptide growth hormone secretagogue, on the growth hormone-insulin-like growth factor I axis in growth hormone-deficient children. Clin Pharmacol. Ther. 2001;70(1):91-8.
Clinical Research
IPS Level of Evidence
IPS Clinical Pharmacists have developed a method of ranking the studies so that the practitioner can easily discern the level of evidence this study provides to the topic. Levels 1-8 are listed below:
| Level of Evidence | Description | |
| Level 1 | FDA Approved Drug studies | |
| Level 2 | Evidence obtained from systematic review and/or meta-analyses of studies including RCTs and other human studies | |
| X | Level 3 | Evidence obtained from a RCT |
| X | Level 4 | Evidence obtained from a study without randomization |
| Level 5 | Evidence obtained from case reports | |
| Level 6 | Evidence obtained from in vitro human studies | |
| X | Level 7 | Evidence obtained from laboratory animal studies |
| X | Level 8 | Evidence obtained from Opinions or Reviews |
Levels
Level 3
Murphy MG, et al. MK-677 an orally active growth hormone secretagogue, reverses diet-induced catabolism. J Clin Endocrinol.1998;83(2):320-25.
Abstract
The reversal of diet-induced negative nitrogen balance by GH suggests a possible therapeutic role for GH treatment in catabolic patients. A double-blind, randomized, placebo-controlled, two-period cross-over study was designed to investigate whether MK-677, an orally active nonpeptide mimic of GH-releasing peptide, can reverse diet-induced protein catabolism. Eight healthy volunteers (ages 24-39 yr) were calorically restricted (18 kcal/kg.day) for two 14-day periods. During the last 7 days of each diet period, subjects received either oral MK-677 25 mg or placebo once daily. There was a 14- to 21-day washout interval between periods. During the first week of caloric restriction (i.e. diet alone), daily nitrogen losses were similar for both treatment groups (mean +/- SE; MK-677 group -2.67 +/- 0.40 g/day vs. placebo group -2.83 +/- 0.26 g/day). During the second week (diet and study drug), mean daily nitrogen balance was 0.31 +/- 0.21 g/day in the MK-677 treatment group compared with -1.48 +/- 0.21 g/day in the placebo group (P < 0.01). MK-677 improved nitrogen balance integrated over the 7 days of treatment; area under the curve day 8-14 nitrogen balance response was +2.69 +/- 5.0 (SE) for MK-677 and -8.97 +/- 5.26 g.day for placebo (P < 0.001). MK-677 produced a peak GH response of 55.9 +/- 31.7 micrograms/L after single dose (day 1 of treatment) and 22.6 +/- 9.3 micrograms/L after a week of dosing compared with placebo treatment peak GH values of approximately 9 (treatment day 1) and approximately 7 micrograms/L (treatment day 7). Following the initial 7-day caloric restriction, insulin-like growth factor-I (IGF-I) declined from 232 +/- 25 to 186 +/- 19 ng/mL in the MK-677 group and from 236 +/- 19 to 174 +/- 23 ng/mL in the placebo group. Mean IGF-I concentration increased significantly during MK-677 to 264 +/- 31 ng/mL (mean for the last 5 days of treatment) compared with 188 +/- 19 ng/mL with placebo (P < 0.01). No significant difference in IGF binding protein-2 was found between the MK-677 and placebo treatments. However, the mean in IGF binding protein-3 for the last 5 days of MK-677 treatment was also significantly increased to 3273 +/- 330 ng/mL (mean +/- SE) compared with placebo 2604 +/- 253 ng/mL (P < 0.01). Neither the serum cortisol nor the PRL response was significantly greater after 7 days of MK-677 dosing compared with 7 days of placebo. MK-677 (25 mg) was generally well tolerated and without clinically significant adverse experiences. In conclusion, MK-677 reverses diet-induced nitrogen wasting, suggesting that if these short-term anabolic effects are maintained in patients who are catabolic because of certain acute or chronic disease states, it may be useful in treating catabolic conditions.
https://sci-hub.se/10.1210/jc.83.2.320
Naas R, et la. Effects of oral ghrelin mimetic on body composition and clinical outcomes in healthy older adults: a randomized, controlled trial. Ann Intern Med. 2008;149(9):601-11.
Abstract
Background: Growth hormone (GH) secretion and muscle mass decline from mid-puberty throughout life culminating in sarcopenia, frailty, decreased function and loss of independence.
Objective: Determine if an oral ghrelin mimetic (MK-677) would enhance GH secretion into the young adult range without serious adverse effects, prevent the decline of fat-free mass (FFM), and decrease abdominal visceral fat (AVF) in healthy older adults.
Design: Two-year, double-blind, randomized, placebo-controlled, modified-crossover clinical trial.
Setting: General Clinical Research Center study performed at a University Hospital.
Participants: Sixty-five healthy men and women (on or off hormone replacement therapy) ages 60-81.
Intervention: Oral administration of MK-677 (25 mg) or placebo once daily.
Measurements: Growth hormone and insulin-like growth factor-I (IGF-I); FFM and AVF were the primary endpoints after one year of treatment. Other endpoints: weight, fat mass, insulin sensitivity, lipid and cortisol levels, bone mineral density, limb lean and fat mass, isokinetic strength, function and quality of life; all endpoints were assessed at baseline and every 6 months.
Limitations: Study design (duration and subject number) not sufficient to evaluate functional endpoints in healthy elderly.
Results: Daily MK-677 significantly increased GH and IGF-I levels to those of healthy young adults without serious adverse effects. With placebo, mean (95% Cl) FFM decreased -0.5 (-1.1 to 0.2) kg, however, FFM increased 1.1 (0.7 to 1.5) kg with MK-677 (P<0.001, MK-677 vs. placebo); body cell mass as reflected by intracellular water decreased -1.0 (-2.1 to 0.2) kg with placebo, but increased 0.8 (-0.1 to 1.6) kg with MK-677 (P=0.021). There were no significant differences in AVF or total fat mass. However, the average increase in limb fat in the MK-677 group (1.1 kg) was greater than with placebo (0.24 kg); P=0.001. Body weight increased 0.8 (-0.3 to 1.8) kg with placebo and 2.7 (2.0 to 3.5) kg with MK-677 (P=0.003). Fasting blood glucose increased an average of 0.3 mmol/L (5 mg/dL) with MK-677 (P=0.015) and insulin sensitivity declined. The most frequent side effects were an increase in appetite that subsided within a few months and transient, mild lower extremity edema and muscle pain. Low density lipoprotein cholesterol decreased -0.14 (-0.27 to -0.01) mmol/L [-5.4 (-10.4 to -0.4) mg/dL] with MK-677 (P=0.026); there were no differences in total or high density lipoprotein cholesterol. Cortisol increased 47 (28 to 71) nmol/L [1.7 (1.0 to 2.6 µg/dL)] with MK-677 (P=0.020). Changes in bone mineral density consistent with increased bone remodeling occurred in MK-677-treated subjects. Increased FFM did not result in changes in strength or function. Two-year exploratory analyses confirmed the 1-year results.
Conclusions: The ghrelin mimetic MK-677 enhanced pulsatile GH secretion and significantly increased FFM over 12 months and was generally well tolerated. Long-term functional, and ultimately pharmaco-economic, studies in elderly adults are indicated.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2757071/pdf/nihms-128599.pdf
Murphy MG, et al. Effect of alendronate and MK-677 (a growth hormone secretagogue), individually and in combination, on markers of bone turnover and bone mineral density in postmenopausal osteoporotic women. J clin Endocrinol Metab. 2001;86(3):1116-25.
Abstract
GH increases bone turnover and stimulates osteoblast activity. We hypothesized that administration of MK-677, an orally active GH secretagogue, together with alendronate, a potent inhibitor of bone resorption, would maintain a higher bone formation rate relative to that seen with alendronate alone, thereby generating greater enhancement of bone mineral density (BMD) in women with postmenopausal osteoporosis. We determined the individual and combined effects of MK-677 and alendronate administration on insulin-like growth factor I levels and biochemical markers of bone formation (osteocalcin and bone-specific alkaline phosphatase) and resorption [urinary N-telopeptide cross-links (NTx)] for 12 months and BMD for 18 months. In a multicenter, randomized, double blind, placebo-controlled, 18-month study, 292 women (64-85 yr old) with low femoral neck BMD were randomly assigned in a 3:3:1:1 ratio to 1 of 4 daily treatment groups for 12 months: MK-677 (25 mg) plus alendronate (10 mg); alendronate (10 mg); MK-677 (25 mg); or a double dummy placebo. Patients who received MK-677 alone or placebo through month 12 received MK-677 (25 mg) plus alendronate (10 mg) from months 12-18. All other patients remained on their assigned therapy. All patients received 500 mg/day calcium. The primary results, except for BMD, are provided for month 12. MK-677, with or without alendronate, increased insulin-like growth factor I levels from baseline (39% and 45%; P < 0.05 vs. placebo). MK-677 increased osteocalcin and urinary NTx by 22% and 41%, on the average, respectively (P < 0.05 vs. placebo). MK-677 and alendronate mitigated the reduction in bone formation compared with alendronate alone based on mean relative changes in serum osteocalcin (-40% vs. -54%; P < 0.05, combination vs. alendronate) and reduced the effect of alendronate on resorption (NTx) as well (-52% vs. -61%; P < 0.05, combination vs. alendronate). MK-677 plus alendronate increased BMD at the femoral neck (4.2% vs. 2.5% for alendronate; P < 0.05). However, similar enhancement was not seen with MK-677 plus alendronate in BMD of the lumbar spine, total hip, or total body compared with alendronate alone. GH-mediated side effects were noted in the groups receiving MK-677, although adverse events resulting in discontinuation from the study were relatively infrequent. In conclusion, the anabolic effect of GH, as produced through the GH secretagogue MK-677, attenuated the indirect suppressive effect of alendronate on bone formation, but did not translate into significant increases in BMD at sites other than the femoral neck. Although the femoral neck is an important site for fracture prevention, the lack of enhancement in bone mass at other sites compared with that seen with alendronate alone is a concern when weighed against the potential side effects of enhanced GH secretion.
https://sci-hub.se/10.1210/jc.86.3.1116
Svensson J, et al. Two-Month Treatment of Obese Subjects with the Oral Growth Hormone (GH) Secretagogue MK-677 Increases GH Secretion, Fat-Free Mass, and Energy Expenditure. J Clin Endocrinol Metabl. 1998;83(2):362-68.
Abstract
The reversal of diet-induced negative nitrogen balance by GH suggests a possible therapeutic role for GH treatment in catabolic patients. A double-blind, randomized, placebo-controlled, two-period cross-over study was designed to investigate whether MK-677, an orally active nonpeptide mimic of GH-releasing peptide, can reverse diet-induced protein catabolism. Eight healthy volunteers (ages 24-39 yr) were calorically restricted (18 kcal/kg.day) for two 14-day periods. During the last 7 days of each diet period, subjects received either oral MK-677 25 mg or placebo once daily. There was a 14- to 21-day washout interval between periods. During the first week of caloric restriction (i.e. diet alone), daily nitrogen losses were similar for both treatment groups (mean +/- SE; MK-677 group -2.67 +/- 0.40 g/day vs. placebo group -2.83 +/- 0.26 g/day). During the second week (diet and study drug), mean daily nitrogen balance was 0.31 +/- 0.21 g/day in the MK-677 treatment group compared with -1.48 +/- 0.21 g/day in the placebo group (P < 0.01). MK-677 improved nitrogen balance integrated over the 7 days of treatment; area under the curve day 8-14 nitrogen balance response was +2.69 +/- 5.0 (SE) for MK-677 and -8.97 +/- 5.26 g.day for placebo (P < 0.001). MK-677 produced a peak GH response of 55.9 +/- 31.7 micrograms/L after single dose (day 1 of treatment) and 22.6 +/- 9.3 micrograms/L after a week of dosing compared with placebo treatment peak GH values of approximately 9 (treatment day 1) and approximately 7 micrograms/L (treatment day 7). Following the initial 7-day caloric restriction, insulin-like growth factor-I (IGF-I) declined from 232 +/- 25 to 186 +/- 19 ng/mL in the MK-677 group and from 236 +/- 19 to 174 +/- 23 ng/mL in the placebo group. Mean IGF-I concentration increased significantly during MK-677 to 264 +/- 31 ng/mL (mean for the last 5 days of treatment) compared with 188 +/- 19 ng/mL with placebo (P < 0.01). No significant difference in IGF binding protein-2 was found between the MK-677 and placebo treatments. However, the mean in IGF binding protein-3 for the last 5 days of MK-677 treatment was also significantly increased to 3273 +/- 330 ng/mL (mean +/- SE) compared with placebo 2604 +/- 253 ng/mL (P < 0.01). Neither the serum cortisol nor the PRL response was significantly greater after 7 days of MK-677 dosing compared with 7 days of placebo. MK-677 (25 mg) was generally well tolerated and without clinically significant adverse experiences. In conclusion, MK-677 reverses diet-induced nitrogen wasting, suggesting that if these short-term anabolic effects are maintained in patients who are catabolic because of certain acute or chronic disease states, it may be useful in treating catabolic conditions.
https://sci-hub.se/10.1210/jcem.83.2.4539
Murphy MG, et al. Oral administration of the growth hormone secretagogue MK-677 increases markers of bone turnover in healthy and functionally impaired elderly adults. The MK-677 Study Group. J Bone Miner Res. 1999;14(7):1182-8.
Abstract
Growth hormone (GH) stimulates osteoblasts in vitro and increases bone turnover and stimulates osteoblast activity when given to elderly subjects. Probably a major effect of GH on bone is mediated through stimulation of either circulating or locally produced insulin-like growth factor I (IGF-I). We determined the effect of chronic administration of the GH secretagogue, MK-677, on serum IGF-I and markers of bone turnover in 187 elderly adults (65 years or older) enrolled in three randomized, double-blind, placebo-controlled clinical studies lasting 2-9 weeks. Urine was collected for determination of N-telopeptide cross-links (NTXs), a marker of bone resorption, and blood was collected for determination of serum osteocalcin and bone-specific alkaline phosphatase (BSAP), as bone formation markers, and serum IGF-I levels pre- and post-treatment. Dose response data were initially obtained in healthy elderly subjects who received oral doses of 10 mg or 25 mg of MK-677 or placebo for 2 weeks (n = 10-12/group). Treatment with 10 mg and 25 mg of MK-677 for 2 weeks increased mean urine NTXs 10% and 17%, respectively (p < 0.05 vs. placebo). Additionally, 50 healthy elderly subjects received either placebo (n = 20) for 4 weeks or 25 mg of MK-677 (n = 30) daily for 2 weeks followed by 50 mg daily for 2 weeks. MK-677 increased mean serum osteocalcin by 8% (p < 0.05 vs. placebo). In both studies, MK-677 increased serum IGF-I levels significantly (55-94%). Subsequently, the biological effects of MK-677 were studied in 105 elderly subjects who met objective criteria for functional impairment. Subjects were randomized to receive oral doses of placebo for 9 weeks or either 5, 10, or 25 mg of MK-677 daily for an initial 2 weeks followed by 25 mg of MK-677 daily for the next 7 weeks(n = 63 on MK-677 and n = 28 on placebo completed 9 weeks of therapy). Treatment with MK-677 (all MK-677 groups combined) for 9 weeks increased mean serum osteocalcin by 29.4% and BSAP by 10.4% (p < 0.001 vs. placebo) and mean urinary NTX excretion by 22.6% (p < 0.05 vs. placebo). The change from baseline serum osteocalcin correlated with the change from baseline serum IGF-I in the MK-677 group (r = 0.37; p < 0.01). In conclusion, once daily dosing with MK-677, an orally active GH secretagogue, stimulates bone turnover in elderly subjects based on elevations in biochemical markers of bone resorption and formation.
https://asbmr.onlinelibrary.wiley.com/doi/epdf/10.1359/jbmr.1999.14.7.1182
Level 4
Chapman IM, et al. Oral administration of growth hormone (GH) releasing peptide-mimetic MK-677 stimulates the GH/insulin-like growth factor-I axis in selected GH-deficient adults. J Clin Endocrinol Metab. 1997;82(10):3455-63.
Abstract
To determine the effect of the GH releasing peptide (GHRP)-mimetic, MK-677, on the GH/insulin-like growth factor-I (IGF-I) axis in selected GH-deficient adults, we studied nine severely GH-deficient men [peak serum GH concentration in response to insulin-induced hypoglycemia of 1.2 6 1.5 mg/L, mean 6 SD (range 0.02–4.79)], age 17–34 yr, height 168 6 1.5 cm, body mass index 22.6 6 3.3 kg/m2 , who had been treated for GH deficiency with GH during childhood. In a double-blind rising-dose design, subjects received once daily oral doses of 10 or 50 mg MK-677 or placebo for 4 days over two treatment periods separated by at least 28 days. Four subjects received placebo and 10 mg/day MK-677 in a cross-over fashion in periods 1 and 2. Five subjects received 10 mg and then 50 mg/day MK-677 in a sequential, rising-dose fashion in periods 1 and 2, respectively. Blood was collected every 20 min for 24 h before treatment and at the end of each period for GH measurement using an ultrasensitive assay. The drug was generally well tolerated, with no significant changes from baseline in circulating concentrations of cortisol, PRL, and thyroid hormones. Serum IGF-I and 24-h mean GH concentrations increased in all subjects after treatment with both 10 and 50 mg/day MK-677 vs. baseline. After treatment with 10 mg MK-677, IGF-I concentrations increased 52 6 20% (65 6 6 to 99 6 9 mg/L, geometric mean 6 intrasubject SE, P # 0.05 vs. baseline), and 24 h mean GH concentrations increased 79 6 19% (0.14 6 0.01 to 0.26 6 0.02 mg/L, P # 0.05 vs. baseline). Following treatment with 50 mg MK-677, IGF-I concentrations increased 79 6 9% (84 6 3 to 150 6 6 mg/L, P # 0.05 vs. baseline) and 24-h mean GH concentrations increased 82 6 29% (0.21 6 0.02 to 0.39 6 0.04 mg/L, P # 0.05 vs. baseline), respectively. Serum IGF binding protein-3 concentrations increased with both 10 mg (1.2 6 0.1 to 1.7 6 0.1 mg/L, P # 0.05) and 50 mg MK-677 (1.7 6 0.1 to 2.2 6 0.2 mg/L, P # 0.05). The GH response to MK-677 was greater in subjects who were the least GH/IGF-I deficient at baseline; by linear regression analysis the increase in 24-h mean GH concentration was positively related to both baseline 24-h mean GH concentration (r 5 0.81, P 5 0.009) and baseline IGF-I (r 5 0.79, P 5 0.01) for 10 mg MK-677. IGF-I responses were not significantly related to any baseline measurement. Fasting and postprandial insulin and postprandial glucose increased significantly after MK-677 treatment, and the clinical significance of these changes will need to be assessed in longer term studies. Oral administration of such GHRP-mimetic compounds may have a role in the treatment of GH deficiency of childhood onset.
https://sci-hub.se/10.1210/jcem.82.10.4297
Codner E, et al. Effects of oral administration of ibutamoren mesylate, a nonpeptide growth hormone secretagogue, on the growth hormone-insulin-like growth factor I axis in growth hormone-deficient children. Clin Pharmacol. Ther. 2001;70(1):91-8.
Abstract
Ibutamoren mesylate (MK-0677), an orally active nonpeptide growth hormone (GH) secretagogue, stimulates GH release through a pituitary and hypothalamic receptor that is different from the GH-releasing hormone receptor. We evaluated the safety and tolerability and the GH-insulin-like growth factor (IGF) responses to two dosages of oral ibutamoren mesylate given to children with GH deficiency for 7 to 8 days. The patients, 18 prepubertal children (15 male, 3 female) with idiopathic GH deficiency, had a chronologic age of 10.6 +/- 0.8 years (mean +/- SD), bone age of 7.4 +/- 0.7 years, growth velocity < 10th percentile for age, height < 10th percentile for age, and a maximum GH response of < or = 10 microg/L to two different GH stimulation tests. The children were assigned as follows to one of three treatment groups with ibutamoren mesylate: 0.2 mg/kg per day for 7 days (days 1-7 or 8-14) and matching placebo for the alternate 7 days (groups I and II, respectively) or 0.8 mg/kg per day for 7 days (days 8-14, group III). On day 15 all patients received an 0.8-mg/kg dose of ibutamoren mesylate. Patients in groups I and II were studied first to assess safety at the low dose before advancement to the high dose. Hormonal profiles were evaluated on day -1 (baseline) and day 15, and the results were expressed as the change from baseline within each group. After administration of ibutamoren mesylate 0.8 mg/kg for 8 days (group III), the median increases (on day 15) from baseline were as follows: 3.8 microg/L (range, 0 to 34.3) for serum GH peak concentration (P = .001), 4.3 microg x h/L (range, 1.3 to 35.6) for the GH area under the concentration-time curve from time zero to 8 hours (AUC(0-8)) (P < .001), 12 microg/L (range, -4 to 116) for serum IGF-I (P = .01), and 0.4 microg/L (range, -0.9 to 1.5) for serum IGF-binding protein 3 (IGFBP-3) (P = .01). There was no change in serum prolactin, glucose, triiodothyronine, thyroxine, thyrotropin, peak serum cortisol, and insulin concentrations or 24-hour urinary free cortisol after administration of 0.8 mg/kg per day of ibutamoren mesylate for 8 days. We conclude that short-term administration of ibutamoren mesylate can increase GH, IGF-I, and IGFBP-3 levels in some children with GH deficiency. Thus this compound is applicable for testing its effect on growth velocity.
https://sci-hub.se/10.1067/mcp.2001.116514
Chapman IM, et al. Stimulation of the growth hormone (GH)-insulin-like growth factor I axis by daily oral administration of a GH secretogogue (MK-677) in healthy elderly subjects. J Clin Endocrinol Metab. 1996;81(12):4249-57.
Abstract
Aging is associated with declining activity of the GH axis, possibly contributing to adverse body composition changes and increased incidence of cardiovascular disease. The stimulatory effects on the GH-insulin-like growth factor I (IGF-I) axis of orally administered MK-677, a GH-releasing peptide mimetic, were investigated. Thirty-two healthy subjects (15 women and 17 men, aged 64-81 yr) were enrolled in a randomized, double blind, placebo-controlled trial. They received placebo or 2, 10, or 25 mg MK-677, orally, once daily for 2 separate study periods of 14 and 28 days. At baseline and on day 14 of each study period, blood was collected every 20 min for 24 h to measure GH, PRL, and cortisol. Attributes of pulsatile GH release were assessed by 3 independent algorithms. MK-677 administration for 2 weeks increased GH concentrations in a dose-dependent manner, with 25 mg/day increasing mean 24-h GH concentration 97 +/- 23% (mean +/- SE; P < 0.05 vs. baseline). This increase was due to an enhancement of preexisting pulsatile GH secretion. GH pulse height and interpulse nadir concentrations increased significantly without significant changes in the number of pulses. With 25 mg/day MK-677 treatment, mean serum IGF-I concentrations increased into the normal range for young adults (141 +/- 21 microgram/L at baseline, 219 +/- 21 micrograms/L at 2 weeks, and 265 +/- 29 micrograms/L at 4 weeks; P < 0.05). MK-677 produced significant increases in fasting glucose (5.4 +/- 0.3 to 6.8 +/- 0.4 mmol/L at 4 weeks; P < 0.01 vs. baseline) and IGF-binding protein-3. Circulating cortisol concentrations did not change, and PRL concentrations increased 23%, but remained within the normal range. Once daily treatment of older people with oral MK-677 for up to 4 weeks enhanced pulsatile GH release, significantly increased serum GH and IGF-I concentrations, and, at a dose of 25 mg/day, restored serum IGF-I concentrations to those of young adults.
No Full Text Available
Copinschi G, et al. Effects of a 7 day treatment with a novel, orally active growth hormone secretagogue, MK-677 on 24 hr GH profiles insulin like growth factor1 and adrenocortical function in normal young men. The Journal of Clinical Endocrinology and Metabolism. 81 (8): 2776–82.
Abstract
To assess the of GH-releasing effects of prolonged administration of a novel analog peptide (MK-6771, nine healthv voune men narticipated in a randomized, double blind, three-periodcros~over cbmparison of orally administered placebo and 5- and 25-mg doses of MK-677. Each period involved bedtime administration of the drug for 7 consecutive days. At the end of each period, plasma levels of insulin-like growth factor I (IGF-I) and IGF-binding protein-3 (IGFBP-3) were measured at 0745 h, and 24-h profiles of plasma GH and cortisol were obtained at 15-min intervals together with the 24-h urinary excretion of free cortisol. Profiles of plasma free cortisol were calculated at hourly intervals. The amounts of GH secreted were similar in all three conditions, but GH pulse frequency was increased with both dosages of the drug, primarily because of an increase in the number of low amplitude pulses. Plasma IGF-I levels were increased in a dose-dependent manner, whereas IGFBP-3 levels were increased only with the highest dosage. There was a positive relationship between GH pulse frequency and IGF-I increase. Except for an advance in the nocturnal nadir and in the morning elevation, MK-677 had no effect on cortisol profiles. In particular, 24-h mean levels of plasma total and free cortisol and urinary excretion of free cortisol were similar under all conditions. The present data suggest that the use of MK-677 for the treatment of relative somatotropic deficiency, particularly in older adults compromised by such deficiency, deserves further investigation.
Level 7
Lee J, et al. Effect of the orally active growth hormone secretagogue MK-677 on somatic growth in rats. Yonsei Med J. 2018;59(10):1174-80.
Abstract
Purpose: Growth hormone secretagogues (GHSs) possess the ability to release growth hormone (GH) in the body. This study aimed to investigate the effects of MK-677, an orally active GHS, on somatic growth in rats.
Materials and Methods: The serum levels of GH were measured after oral administration of MK-677 to confirm GH stimulatory effects. Body weight, body length, tibia length, epiphyseal plate width, and serum levels of insulin-like growth factor (IGF)-I were measured after oral administration of 4 mg/kg of MK-677 for 6 weeks to investigate growth-promoting effects.
Results: Oral administration of MK-677 at 4 mg/kg increased peak GH concentrations by 1.8-fold, compared to baseline. However, oral administration of MK-677 for 6 weeks did not increase body growth or serum levels of IGF-I. At 6 weeks after treatment, the GH response to MK-677 was abolished. Pituitary GH mRNA and hypothalamic GH-releasing hormone mRNA, and GH secretagogue receptor (GHSR) mRNA expression in the pituitary and hypothalamus did not differ between the control and treatment group. Somatostatin (SST) mRNA expression in the hypothalamus was markedly increased in the treatment group, whereas SST receptor (SSTR)-2 mRNA expression in the pituitary gland was decreased. Protein expression of hypothalamic GHSR, SST, and pituitary SSTR-2 showed patterns similar to those for mRNA expression.
Conclusion: Our results suggest that prolonged administration of MK-677 in rats does not promote growth despite the GH stimulatory effect of MK-677, which may be related to increased expression of SST in the hypothalamus. Further studies are needed to overcome the observed desensitization to GHS.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6240568/pdf/ymj-59-1174.pdf
Jeong YO, et al. MK-0677, a Ghrelin Agonist, Alleviates Amyloid Beta-Related Pathology in 5XFAD Mice, an Animal Model of Alzheimer’s Disease. Int J Mol Sci. 2018;19(6):1800.
Abstract
Alzheimer’s disease (AD) is a progressive neurodegenerative disorder characterized by cognitive deficits, neuroinflammation, and neuronal death. The primary pathogenic cause is believed to be the accumulation of pathogenic amyloid beta (Aβ) assemblies in the brain. Ghrelin, which is a peptide hormone predominantly secreted from the stomach, is an endogenous ligand for the growth hormone secretagogue-receptor type 1a (GHS-R1a). MK-0677 is a ghrelin agonist that potently stimulates the GHS-R1a ghrelin receptor. Interestingly, previous studies have shown that ghrelin improves cognitive impairments and attenuates neuronal death and neuroinflammation in several neurological disorders. However, it is unknown whether MK-0677 can affect Aβ accumulation or Aβ-mediated pathology in the brains of patients with AD. Therefore, we examined the effects of MK-0677 administration on AD-related pathology in 5XFAD mice, an Aβ-overexpressing transgenic mouse model of AD. MK-0677 was intraperitoneally administered to three-month-old 5XFAD mice. To visualize Aβ accumulation, neuroinflammation, and neurodegeneration, thioflavin-S staining and immunostaining with antibodies against Aβ (4G8), ionized calcium-binding adaptor molecule 1 (Iba-1), glial fibrillary acidic protein (GFAP), neuronal nuclear antigen (NeuN), and synaptophysin were conducted in the neocortex of 5XFAD and wild-type mice, and to evaluate changes of phosphorylated cyclic adenosine monophosphate (cAMP) response element binding protein (pCREB) levels, immunostaining with antibody against pCREB was performed in dentate gyrus of the hippocampus of 5XFAD and wild-type mice. The histological analyses indicated that MK-0677-treated 5XFAD mice showed reduced Aβ deposition, gliosis, and neuronal and synaptic loss in the deep cortical layers, and inhibited the decrement of pCREB levels in dentate gyrus of the hippocampus compared to vehicle-treated 5XFAD mice. Our results showed that activation of the ghrelin receptor with MK-0677 inhibited the Aβ burden, neuroinflammation, and neurodegeneration, which suggested that MK-0677 might have potential as a treatment of the early phase of AD.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6032329/pdf/ijms-19-01800.pdf
Tian J, et al. MK0677, a Ghrelin Mimetic, Improves Neurogenesis but Fails to Prevent Hippocampal Lesions in a Mouse Model of Alzheimer’s Disease Pathology. J Alzheimers dis. 2019;72(2):467-78.
Abstract
Hippocampal lesions including synaptic injury, neuroinflammation, and impaired neurogenesis are featured pathology closely associated with neuronal stress and cognitive impairment in Alzheimer’s disease (AD). Previous studies suggest that ghrelin and its receptor, growth hormone secretagogue receptor 1α (GHSR1α), promote hippocampal synaptic function and neurogenesis. GHSR1α activation thus holds the potential to be a therapeutic avenue for the treatment of hippocampal pathology in AD; however, a comprehensive study on the preventive effect of MK0677 on hippocampal lesions in AD-related conditions is still lacking. In this study, we treated a transgenic mouse model of AD-like amyloidosis (5xFAD mice) at the asymptomatic stage with MK0677, a potent ghrelin mimetic. We found that MK0677 fostered hippocampal neurogenesis in 5xFAD mice but observed little preventive function with regards to the development of hippocampal amyloid-β (Aβ) deposition, synaptic loss, microglial activation, or cognitive impairment. Furthermore, MK0677 at a dose of 3 mg/kg significantly increased 5xFAD mouse mortality. Despite enhanced hippocampal neurogenesis, MK0677 treatment has little beneficial effect to prevent hippocampal lesions or cognitive deficits against Aβ toxicity. This study, together with a failed large-scale clinical trial, suggests the ineffectiveness of MK0677 alone for AD prevention and treatment.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7153492/pdf/nihms-1571717.pdf
Level 8
Ishida J, et al. Growth hormone secretagogues: history, mechanism of action and clinical development. JCSM Rapid Comm. 2020;
Abstract
Growth hormone secretagogues (GHSs) are a generic term to describe compounds that increase growth hormone (GH) re-lease. GHSs include agonists of the growth hormone secretagogue receptor (GHS-R), whose natural ligand is ghrelin, and agonists of the growth hormone-releasing hormone (GHRH) receptor, to which the GHRH binds as a native ligand. Several GHSs have been developed with a view to treating or diagnosing of GH deficiency, which causes growth retardation, gastrointestinal dysfunction, and altered body composition, in parallel with extensive research to identify GHRH, GHS-R, and ghrelin. This re-view will focus on the research history and the pharmacology of each GHS, which reached randomized clinical trials. Further-more, we will highlight the publicly disclosed clinical trials regarding GHSs.
[1] Murphy MG, et al. MK-677 an orally active growth hormone secretagogue, reverses diet-induced catabolism. J Clin Endocrinol.1998;83(2):320-25.
[1] Svensson J, et al. Two-Month Treatment of Obese Subjects with the Oral Growth Hormone (GH) Secretagogue MK-677 Increases GH Secretion, Fat-Free Mass, and Energy Expenditure. J Clin Endocrinol Metabl. 1998;83(2):362-68.
[1] Patchett AA, et al. Design and biological activities of L-163,191 (MK-0677): a potent, orally active growth hormone secretagogue. Proc Natl Acad Sci USA. 1995;92:7001-5.
[1] Patchett AA, et al. Design and biological activities of L-163,191 (MK-0677): a potent, orally active growth hormone secretagogue. Proc Natl Acad Sci USA. 1995;92:7001-5.
[1] Copinschi G, et al. Effects of a 7 day treatment with a novel, orally active growth hormone secretagogue, MK-677 on 24 hr GH profiles insulin like growth factor1 and adrenocortical function in normal young men. The Journal of Clinical Endocrinology and Metabolism. 81 (8): 2776–82.
[1] Naas R, et la. Effects of oral ghrelin mimetic on body composition and clinical outcomes in healthy older adults: a randomized, controlled trial. Ann Intern Med. 2008;149(9):601-11.
[1] Murphy MG, et al. Oral administration of the growth hormone secretagogue MK-677 increases markers of bone turnover in healthy and functionally impaired elderly adults. The MK-677 Study Group. J Bone Miner Res. 1999;14(7):1182-8.
[1] Murphy MG, et al. Effect of alendronate and MK-677 (a growth hormone secretagogue), individually and in combination, on markers of bone turnover and bone mineral density in postmenopausal osteoporotic women. J clin Endocrinol Metab. 2001;86(3):1116-25.
[1] Ibutamoren – Lumos Pharma/Merck – AdisInsight”.
[1] Adunsky A, et al. MK-0677 (ibutamoren mesylate) for the treatment of patients recovering from hip fracture: a multicenter, randomized, placebo-controlled phase Iib study. Arch Gerontol Geriatr. 2011;53(2):183-9.
[1] Svensson J, et al. Treatment with the oral growth hormone secretagogue MK-677 increases markers of bone formation and bone resorption in obese young males. J Bone Miner Res. 1998;13(7):1158-66.
[1] Codner E, et al. Effects of oral administration of ibutamoren mesylate, a nonpeptide growth hormone secretagogue, on the growth hormone-insulin-like growth factor I axis in growth hormone-deficient children. Clin Pharmacol. Ther. 2001;70(1):91-8.