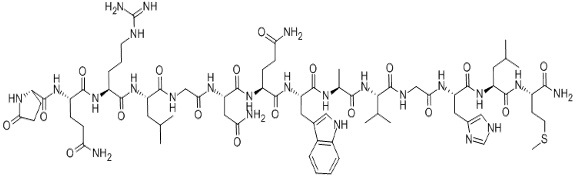Bombesin
GASTRIN-RELEASING PEPTIDE (GRP)
Contents
Most Frequent Uses:
- Regulates Gastrointestinal hormone release and GI motility
- Modulate satiety
Dosage:
- General Dosage
- None Established
Safety and Potential Side Effects/Contraindications:
- Bombesin BBN upregulation of GRP is associated with cancers including: Breast cancer; Colon cancer; Gastric cancer; Glioblastoma; Pancreatic cancer; Prostate cancer; Small cell lung cancer
- BBN receptors expressed in cancer cells exhibit mitogenic effects, stimulating tumor cell proliferation when activated by agonistic molecules.
- Use in cancer is not recommended until further research can be performed.
Description
Bombesin (BBN) is a neuropeptide primarily isolated from the skin of the European fire-bellied toad, Bombina bombina. The peptide is composed of 14 amino acid residues and carries a natural carboxyl-terminal (C-terminal) carboxyamide. It is homolog to the mammalian gastrin-releasing peptide (GRP), exhibiting high similarity in their C-terminal portion.
Bombesin binds to and activates G-protein coupled receptors, known as gastrin releasing peptide receptor (GRPR). Bombesin displays many physiological effects, including smooth muscle contraction, promotion of cell growth, and mediation of endocrine responses, like inducing gastrin release, and is implicated in control some aspects of behavior, including feeding.
Recently, studies have discussed the role of bombesin in tumor growth, cellular proliferation, and inflammation.[i] Research has also discovered several peptides structurally related to bombesin.[ii] Two well-studied homologs are called neuromedin B and gastrin-releasing peptide (GRP).[iii] The gastrin-releasing peptide is biologically and immunologically equivalent to bombesin, making GRP the mammalian equivalent. In addition to gastric neurohormonal impacts on the GI tract, the BN-like peptides have also been shown to modulate satiety, thermoregulation, glucose homeostasis, and circadian rhythms.[iv],[v]
Four BBN receptor subtypes have been described to-date: [vi]
- Neuromedin B receptor (BB1r)
- Gastrin-releasing peptide receptor (BB2r or GRPr)
- Orphan receptor (BB3r),
- Amphibious receptor (BB4r)
The latter is found in amphibians, while the others are presented in mammalian cells. They are all G-protein coupled receptors and their pharmacological activities include the stimulation of hormones releasing, like gastrin and somatostatin, as well as the stomach and intestine smooth muscle contraction.
Bombesin and its analogues have been implicated as autocrine and/or paracrine growth factors in human lung carcinoma. [vii] Human bombesin receptors, GRPR and NMBR, are two of the most frequently overexpressed G-protein-coupled-receptors by lung-cancers. Recently, GRPR/NMBR are receiving considerable attention because they act as growth factor receptors often in an autocrine manner in different lung-cancers, affect tumor angiogenesis, their inhibition increases the cytotoxic potency of tyrosine-kinase inhibitors reducing lung-cancer cellular resistance/ survival and their overexpression can be used for sensitive tumor localization as well as to target cytotoxic agents to the cancer.[viii] The orphan BRS-3-receptor, because of homology is classified as a bombesin receptor but has received little attention, despite the fact that it is also reported in a number of studies in lung-cancer cells and has growth effects in these cells.
Clinical Research
IPS Level of Evidence
IPS Clinical Pharmacists have developed a method of ranking the studies so that the practitioner can easily discern the level of evidence this study provides to the topic. Levels 1-8 are listed below:
| Level of Evidence | Description | |
| Level 1 | FDA Approved Drug studies | |
| Level 2 | Evidence obtained from systematic review and/or meta-analyses of studies including RCTs and other human studies | |
| Level 3 | Evidence obtained from a RCT | |
| Level 4 | Evidence obtained from a study without randomization | |
| Level 5 | Evidence obtained from case reports | |
| X | Level 6 | Evidence obtained from in vitro human studies |
| X | Level 7 | Evidence obtained from laboratory animal studies |
| Level 8 | Evidence obtained from Opinions or Reviews |
Levels
Level 6
Fathi Z, et al. Bombesin receptor structure and expression in human lung carcinoma cell lines. J Cell Biochem Suppl. 1996;24:237-46.
Abstract
Mammalian bombesin-like peptides gastrin-releasing peptide (GRP) and neuromedin B (NMB) are regulatory neuropeptides involved in numerous physiologic processes, and have been implicated as autocrine and/or paracrine growth factors in human lung carcinoma. Three structurally and pharmacologically distinct hombesin receptor subtypes have been isolated and characterized: the gastrin releasing peptide receptor (GRP-R), the neuromedin B receptor (NMB-R), and bombesin receptor subtype-3 (BRS-3). The three receptors are structurally related, sharing about 50% amino acid identity. They are members of the G-protein coupled receptor superfamily with a seven predicted transmembrane segment topology characteristic of receptors in this family. The signal transduction pathway for GRP-R and NMB-R involves coupling to a pertussis-toxin insensitive G-protein, activation of phospholipase C (PLC), generation of inositol trisphosphate (IP3), release of intracellular calcium, and activation of protein kinase C. While all three bombesin receptors are activated by bombesin agonists, GRP-R, NMB-R, and BRS-3 have very different affinities for the mammalian bombesin-like peptides GRP and NMB, as well as bombesin receptor antagonists. The three bombesin receptor subtypes are expressed in an overlapping subset of human lung carcinoma cell lines. Any therapeutfc strategy based on modulation of bombesin growth responses in human lung carcinoma would be well served to take into account the pharmacologic heterogeneity of the relevant receptors.
https://sci-hub.se/10.1002/jcb.240630519
Accardo A, et al. Easy formulation of liposomal doxorubicin modified with a bombesin peptide analogue for selective targeting of GRP receptors overexpressed by cancer cells. Drug Deliv Transl Res. 2019 Feb;9(1):215-226.
Abstract
The article concerns the obtainment of liposomal doxorubicin (Dox) in which liposomes are externally modified with a targeting peptide able to drive the formulation in a selective way on membrane receptors overexpressed in tumors. We developed a kit composed by three different vials: (A) a vial containing a sterile, translucent, red dispersion of the liposomal doxorubicin drug (Doxil®), (B) a vial filled with a lyophilized powder of a modified phospholipid with a reactive function (DSPE-Peg-maleimide), and (C) a vial containing a 1-9 bombesin peptide analogue (Cys-BN-AA1) chemically modified to react in stoichiometric ratio respect to DSPE-Peg-maleimide. The chosen peptide is a stable analogue antagonist of the wild-type 1-9 bombesin peptide; it is very stable in serum; maintains high specificity, with nanomolar affinity, towards gastrin release peptide receptors (GRPRs indicated also as BB2); and is overexpressed in some cancer cells. Results on animal studies clearly indicate that in mice treated with the kit product (i.e., pegylated liposomal Dox modified with the bombesin analogue, Doxil-BN-AA1), tumor growth is reduced, with an improved efficacy respect to mice treated with non-modified pegylated liposomal Dox or with saline solution.
https://sci-hub.se/10.1007/s13346-018-00606-x
Moreno P, et al. A possible new target in lung-cancer cells: The orphan receptor, bombesin receptor subtype-3. Peptides. 2018 Mar;101:213-226.
Abstract
Human bombesin receptors, GRPR and NMBR, are two of the most frequently overexpressed G-protein-coupled-receptors by lung-cancers. Recently, GRPR/NMBR are receiving considerable attention because they act as growth factor receptors often in an autocrine manner in different lung-cancers, affect tumor angiogenesis, their inhibition increases the cytotoxic potency of tyrosine-kinase inhibitors reducing lung-cancer cellular resistance/survival and their overexpression can be used for sensitive tumor localization as well as to target cytotoxic agents to the cancer. The orphan BRS-3-receptor, because of homology is classified as a bombesin receptor but has received little attention, despite the fact that it is also reported in a number of studies in lung-cancer cells and has growth effects in these cells. To address its potential importance, in this study, we examined the frequency/relative quantitative expression of human BRS-3 compared to GRPR/NMBR and the effects of its activation on cell-signaling/growth in 13 different human lung-cancer cell-lines. Our results showed that BRS-3 receptor is expressed in 92% of the cell-lines and that it is functional in these cells, because its activation stimulates phospholipase-C with breakdown of phosphoinositides and changes in cytosolic calcium, stimulates ERK/MAPK and stimulates cell growth by EGFR transactivation in some, but not all, the lung-cancer cell-lines. These results suggest that human BRS-3, similar to GRPR/NMBR, is frequently ectopically-expressed by lung-cancer cells in which, it is functional, affecting cell signaling/growth. These results suggest that similar to GRPR/NMBR, BRS-3 should receive increased attention as possible approach for the development of novel treatments and/or diagnosis in lung-cancer.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6159918/pdf/nihms-942522.pdf
Level 7
McDonald TJ, et al. Characterization of a gastrin releasing peptide from porcine non-antral gastric tissue. Biochem Biophys Res Commun. 1979 Sep 12;90(1):227-33.
Abstract
Mammalian bombesin-like peptides gastrin-releasing peptide (GRP) and neuromedin B (NMB) are regulatory neuropeptides involved in numerous physiologic processes, and have been implicated as autocrine and/or paracrine growth factors in human lung carcinoma. Three structurally and pharmacologically distinct bombesin receptor subtypes have been isolated and characterized: the gastrin releasing peptide receptor (GRP-R), the neuromedin B receptor (NMB-R), and bombesin receptor subtype-3 (BRS-3). The three receptors are structurally related, sharing about 50% amino acid identity. They are members of the G-protein coupled receptor superfamily with a seven predicted transmembrane segment topology characteristic of receptors in this family. The signal transduction pathway for GRP-R and NMB-R involves coupling to a pertussis-toxin insensitive G-protein, activation of phospholipase C (PLC), generation of inositol trisphosphate (IP3), release of intracellular calcium, and activation of protein kinase C. While all three bombesin receptors are activated by bombesin agonists, GRP-R, NMB-R, and BRS-3 have very different affinities for the mammalian bombesin-like peptides GRP and NMB, as well as bombesin receptor antagonists. The three bombesin receptor subtypes are expressed in an overlapping subset of human lung carcinoma cell lines. Any therapeutic strategy based on modulation of bombesin growth responses in human lung carcinoma would be well served to take into account the pharmacologic heterogeneity of the relevant receptors.
https://sci-hub.se/10.1002/jcb.240630519
Anastasi A, et al. Isolation and amino acid sequences of alytesin and bombesin, two analogous active tetradecapeptides from the skin of European discoglossid frogs. Arch Biochem Biophys. 1972 Feb;148(2):443-6.
Abstract
Crude methanol extracts of fresh skins of the European discoglossid frogs Alytes obstetricans, Bombina bombina, and Bombina variegata were subjected to chromatography on alumina columns and to gel filtration on Sephadex columns. The active constituents of these extracts, the peptides dytesin and bombesin, were obtained in pure form. Acid hydrolysis, digestion with trypsin and chymotrypsin, and group determination experiments demonstrated that alytesin and bombesin are tetradecapeptides differing only in the second and sixth residues from the NH*-terminus. Synthesis, carried out only for bombesin, has confirmed the proposed sequence. The similarity between the structure of alytesin and bombesin on the one hand and that of ranatensin from Rana pipiens on the other is noted and discussed.
https://sci-hub.se/10.1016/0003-9861(72)90162-2
Assimakopoulos SF, et al. Effect of bombesin and neurotensin on gut barrier function in partially hepatectomized rats. World J Gastroenterol. 2005 Nov 21;11(43):6757-64.
Abstract
Aim: To investigate the effect of regulatory peptides bombesin (BBS) and neurotensin (NT) on intestinal barrier function in partially hepatectomized rats.
Methods: Ninety male Wistar rats were randomly divided into five groups: I (n=0): controls, II (n= 20): sham operated, III (n=20): partial hepatectomy 70% (PHx), IV (n=20): PHx+BBS (30 microg/kg/d), V (n=20): PHx+NT (300 microg/kg/d). Groups IV and V were treated for 8 days before PHx and 48 h post surgery. At the end of the experiment, on day 10, intestinal barrier function was assessed by measuring endotoxin concentrations in portal and aortic blood. Tissue sections of the terminal ileum were examined histologically and villus density, mucosal thickness, mitotic activity and apoptosis in crypts were assessed. In addition, ileal mucosa was analyzed for DNA and protein content and microbiological analysis was performed in cecal contents. To estimate intestinal oxidative stress, lipid peroxidation was determined on tissue homogenates from terminal ileum.
Results: BBS or NT administration significantly reduced portal and systemic endotoxemia observed 48 h after partial hepatectomy. In hepatectomized rats (group III), a trend towards induction of mucosal atrophy was observed, demonstrated by the reduction of villus density, mucosal thickness, protein content and significant reduction of DNA, while these alterations were reversed by regulatory peptides administration. This trophic effect of BBS and NT was accompanied by induction of mitoses above control levels and a significant reduction of apoptosis in intestinal crypts. Intestinal lipid peroxidation was found significantly lower in PHx group and regulatory peptides exerted an antioxidant action, further decreasing this parameter of oxidative stress. The bacterial population of E. coli and aerobic Gram (+) cocci was increased in cecal content of hepatectomized rats, while this parameter was not affected by the administration of BBS or NT.
Conclusion: Gut regulatory peptides BBS and NT improve intestinal barrier function and reduce endotoxemia in experimental partial hepatectomy. This effect is, at least in part, mediated by their trophic, anti-apoptotic, mitogenic, and antioxidant effect on the intestinal epithelium. This observation might be of potential value in patients undergoing liver resection.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4725030/pdf/WJG-11-6757.pdf
Giladi E, et al. Molecular cloning and characterization of receptors for the mammalian bombesin-like peptides. J Mol Neurosci. 1993;4(1):41-54.
Abstract
The bombesin-like peptides comprise a large family of peptides common to both amphibians and mammals that function as growth factors, neurotransmitters, and paracrine hormones. GRP, the mammalian homolog of bombesin and its receptor, as well as NMB, the mammalian homolog of ranatensin, are expressed in human neoplasms and, in particular, in small cell lung carcinomas (SCLC). To better characterize the physiological roles of bombesin-like peptides, our laboratory has cloned the receptors for GRP in murines, rats, and humans. The 3T3 GRP receptor was isolated and characterized using the two-electrode-voltage-clamp analysis and acquorin-emission methods in xenopus oocytes expression system. The rat and human GRP and NMB receptors were cloned by hybridization at low stringency, using the mouse cDNA receptor probe. Sequence analysis of the receptors showed 384 and 390 amino acids for GRP and NMB receptors, respectively. The homology between the two receptors is 60% and between species in the same receptor, 90%. The receptors belong to the 7-membrane spanning domains superfamily. The specific GRP-R antagonist blocked the response to bombesin in oocytes injected with GRP-R, but failed to do so in oocytes injected with NMB-R. The two receptors differ in their distribution of tissue expression. RNA blot and RNase protection analysis showed the same size of mRNA without alteration in the receptors. RT + PCR analysis performed on genomic DNA revealed similarity between normal and cell DNAs, suggesting no major gene deletion or rearrangement. Southern blot analysis indicated the absence of gene amplification. Sequence analysis of the exonic segments of the receptor genes displayed identical amino acids to the respective cDNAs. None of the genes had classic TATAA box. Somatic cell hybrids localized the GRP-R on the X-chromosome and the NMB-R on chromosome 6. The same sequence of normal genes and cDNAs of GRP and NMB receptors, together with the gene characterization, demonstrated that SCLC cell lines do not require a structural change in receptor protein or genomic rearrangement.
[i] Moreno P, et al. A possible new target in lung-cancer cells: The orphan receptor, bombesin receptor subtype-3. Peptides. 2018 Mar;101:213-226.
[ii] McDonald TJ, et al. Characterization of a gastrin releasing peptide from porcine non-antral gastric tissue. Biochem Biophys Res Commun. 1979 Sep 12;90(1):227-33.
[iii] Anastasi A, et al. Isolation and amino acid sequences of alytesin and bombesin, two analogous active tetradecapeptides from the skin of European discoglossid frogs. Arch Biochem Biophys. 1972 Feb;148(2):443-6.
[iv] Accardo A, et al. Easy formulation of liposomal doxorubicin modified with a bombesin peptide analogue for selective targeting of GRP receptors overexpressed by cancer cells. Drug Deliv Transl Res. 2019 Feb;9(1):215-226.
[v] Assimakopoulos SF, et al. Effect of bombesin and neurotensin on gut barrier function in partially hepatectomized rats. World J Gastroenterol. 2005 Nov 21;11(43):6757-64.
[vi] LaPelusa A, et al. Biochemistry, Bombesin. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2022 Jan.2021 Aug 1.
[vii] Giladi E, et al. Molecular cloning and characterization of receptors for the mammalian bombesin-like peptides. J Mol Neurosci. 1993;4(1):41-54.
[viii] Moreno P, et al. A possible new target in lung-cancer cells: The orphan receptor, bombesin receptor subtype-3. Peptides. 2018 Mar;101:213-226.