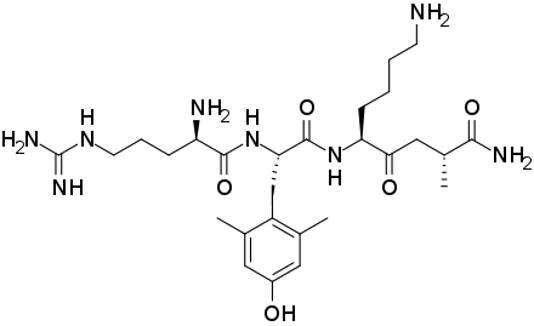
SS31
Elamipretide
Bendavia
MTP-131
Contents
Most Frequent Uses:
- Antioxidant/antiinflammatory
- Mitochondrial protection/ stabilizes cardiolipin
- Anti-aging
- Neurological protection / cognitive/memory support
- Alzheimer’s Disease support
- Brain injury support
- Musculoskeletal injury support
- Cardiomyopathy support
- Kidney support
- Ophthalmic – atrophy in dry Aged-related macular degeneration support (AMD)
Dosage:
- SubQ
- 40 mg SubQ x 12-24 weeks
- Oral
- 10-50 mg daily
Safety and Potential Side Effects/Contraindications:
- SS-31 peptide administered subcutaneously are reported safe in recommended dosages.
- As with all injections, redness, and pain at the site of injection may be present.
Description
SS-31 (also known as Bendavia, Elamipretide, and MTP-131) is a small peptide that accumulates in mitochondria and scavenges reactive oxygen species. SS-31 is a member of the Szeto-Schiller (SS) peptides known to selectively target the inner mitochondrial membrane.
SS31 is reported to improve mitochondrial function and overall production of energy via ATP synthesis. SS31 binds to cardiolipin, a lipid exclusively expressed on the inner mitochondrial membrane that plays an important structural role in organizing the components of the electron transport chain into “supercomplexes” for more efficient oxidative phosphorylation with minimal generation of reactive oxygen species.[i],[ii] By binding to cardiolipin, SS-31 modulates the hydrophobic interaction between cytochrome c and cardiolipin and promotes the electron carrying function of cytochrome c. SS-31 also inhibits the opening of the mitochondrial permeability transition pore that forms under mitochondrial stress (e.g., TBI, stroke, Alzheimer’s/Parkinson’s and other neurodegenerative diseases).[iii]
In preclinical or clinical studies, SS-31 is reported to increase mitochondrial respiration, improve electron transport chain function and ATP production and reduce formation of pathogenic ROS levels.[iv] The SS-31-cardiolipin association is reported to normalize the structure of the inner mitochondrial membrane, thereby improving mitochondrial function, leading to improvement in ATP production and interruption and potential reversal of damaging oxidative stress.[v]
Aging and related health concerns:
Preclinical studies suggest benefits in muscle aging, atherosclerosis, ischemia, osteoarthritis, diabetes, and glaucoma. But clinical trials in heart failure and primary mitochondrial myopathy have failed.
Types of evidence:
- 1 phase 3 double-blind randomized controlled trial in primary mitochondrial myopathy
- 1 phase 2/3 randomized controlled trial in Barth syndrome patients
- 1 double-blind phase 2 RCT in patients with heart failure
- 1 double-blind phase 2 RCT in patients with primary mitochondrial myopathy
- 1 double-blind phase 1/2 RCT in patients with primary mitochondrial myopathy
- 1 double-blind phase 2a RCT in patients with myocardial infarction
- 2 double-blind randomized controlled trials, 1 in patients with atherosclerotic renal artery stenosis and 1 in heart failure
- 1 pilot clinical study in renovascular hypertensive patients undergoing renal revascularization
- Numerous preclinical studies
AMD (Age-related Macular Degeneration)
SS-31 reduces retinal cell death by inhibiting retinal cell apoptosis, by activating retinal proteins and enhancing antioxidative enzyme activity.[vi],[vii] Antioxidant properties lessens the mitochondrial dysfunction in these cells and makes SS-31 a possible treatment for glaucoma and age‑associated retinal diseases.[viii] SS-31 also is reported to have a neuroprotective effect in the retina against diabetes-induced retinal degeneration.[ix]
Type 2 Diabetes
Type 2 diabetes is associated with increased production of reactive oxygen species (ROS), oxidative stress, mitochondrial dysfunction and inflammation. SS-31 repairs issues with the antioxidant system, mitochondria function and levels of inflammatory molecules.[x] SS-31 exerts beneficial effects on the leukocytes of T2D patients by reducing oxidative stress, leukocyte-endothelium interactions, NFκB and TNFα and by increasing SIRT1 levels.
Cardiovascular Health
SS-31 supports decreased oxidative stress in the cardiovascular system. In mice studies, SS-031 protects against cardiomyopathy by improving cardiac hypertrophy, diastolic dysfunction and the formation of scar tissue, which in excess can become pathological.[xi] In addition, SS-31 is reported to prevent the conversion of macrophages to pro-atherogenic foam cells.
Kidney Disease
In chronic kidney disease, the mitochondria in kidney cells are small and rounded, indicative of mitochondrial damage. SS-31 is reported to restore normal mitochondrial structure in kidney cells and suppress further mitochondrial damage.[xii] In lab animal studies, treatment of renal ischemia with SS-31 accelerated ATP recovery upon reperfusion. SS-31 repaired mitochondria in kidney cells and restored their cellular structure after only 1 one month of treatment post-acute injury. Authors reported treatment with SS-31 in acute renal ischemia significantly accelerated the rate of kidney cell regeneration.[xiii]
Cognitive Function & Neuroinflammation
In animal studies, SS-31 is reported to have neuroprotective activity against neurodegenerative disorders, including TBI, amyotrophic lateral sclerosis (ALS), Alzheimer’s and Parkinson’s diseases.[xiv] Neuroprotection by SS-31 is through antioxidant activity, mitochondrial protection and anti-inflammatory activity.
LPS (lipopolysaccharide)-induced memory impairment was significantly reduced by SS-31 by decreasing hippocampal oxidative stress, improving BDNF signaling, improving synapse function, decreasing neuronal death via apoptosis and ultimately improving impaired memory.[xv]
Anti-Aging – Other
Clinical trials with SS-31 have reported promising results in age-related conditions including heart failure, muscle weakness, chronic kidney disease and age-related macular degeneration.[xvi] SS-31 is rapidly taken up by skeletal muscles and ATP production levels returned to those seen in much younger mice within just one hour of SS-31 treatment. The improvement in ATP is associated with improved fatigue resistance.
In another study on mice, leg muscle exercise tolerance in older mice given SS-31 became stronger and less fatigued, with significantly greater mass compared to older mice not given SS-31.[xvii] This also led to a significant increase in treadmill endurance compared to controls not given SS-31.
Clinical Research
IPS Level of Evidence
IPS Clinical Pharmacists have developed a method of ranking the studies so that the practitioner can easily discern the level of evidence this study provides to the topic. Levels 1-8 are listed below:
| Level of Evidence | Description | |
| X | Level 1 | FDA Approved Drug studies |
| Level 2 | Evidence obtained from systematic review and/or meta-analyses of studies including RCTs and other human studies | |
| X | Level 3 | Evidence obtained from a RCT |
| X | Level 4 | Evidence obtained from a study without randomization |
| X | Level 5 | Evidence obtained from case reports |
| X | Level 6 | Evidence obtained from in vitro human studies |
| X | Level 7 | Evidence obtained from laboratory animal studies |
| X | Level 8 | Evidence obtained from Opinions or Reviews |
Levels
Level 1
Allingham MJ, et al. Phase 1 Clinical Trial of Elamipretide in Intermediate Age-Related Macular Degeneration and High-Risk Drusen: ReCLAIM High-Risk Drusen Study
Abstract
Purpose: To assess safety, tolerability, and feasibility of subcutaneous administration of the mitochondrial-targeted drug elamipretide in patients with intermediate age-related macular degeneration (AMD) and high-risk drusen (HRD) and to perform exploratory analyses of change in visual function.
Design: Phase 1, single-center, open-label, 24-week clinical trial with preplanned HRD cohort.
Participants: Adult patients ≥55 years of age with intermediate AMD and HRD.
Methods: Participants received subcutaneous elamipretide 40 mg daily, with safety and tolerability assessed throughout the study. Ocular assessments included normal-luminance best-corrected visual acuity (BCVA), low-luminance best-corrected visual acuity (LLVA), normal-luminance binocular reading acuity (NLRA), low-luminance binocular reading acuity (LLRA), spectral-domain OCT, fundus autofluorescence (FAF), mesopic microperimetry, dark adaptation, and low-luminance questionnaire (LLQ).
Main Outcome Measures: The primary end point was safety and tolerability. Prespecified exploratory end points included changes from baseline in BCVA, LLVA, NLRA, LLRA, retinal pigment epithelium (RPE)-drusen complex (DC) volume by OCT, FAF, mesopic microperimetry, dark adaptation, and LLQ results.
Results: Subcutaneous administration of elamipretide was highly feasible. All participants with HRD (n = 21) experienced 1 or more adverse events (AEs), but all were mild (57%) or moderate (43%), with the most common events related to injection site reactions. No serious systemic AEs occurred. One participant discontinued because of injection site reaction, 1 participant withdrew because they did not wish to continue study visits, and 1 participant withdrew after experiencing transient visual impairment. Among the 18 participants who completed the study, mean change in BCVA from baseline to 24 weeks was +3.6 letters (P = 0.014) and LLVA was +5.6 letters (P = 0.004). Compared with baseline, mean NLRA improved by –0.11 logarithm of the minimum angle of resolution (logMAR) units (P = 0.001), and LLRA by −0.28 logMAR units (P < 0.0001). Significant improvements were found in 6 of 7 subscales of the LLQ (P<0.0015). No significant changes were observed for RPE-DC volume, FAF, mesopic microperimetry, or dark adaptation.
Conclusions: Elamipretide appeared to be generally safe and well tolerated in treating intermediate AMD and HRD. Exploratory analyses demonstrate a positive effect on visual function, particularly under low-luminance conditions. Further study of elamipretide for treatment of intermediate AMD with HRD is warranted.
Mettu PS, et al. Phase 1 Clinical Trial of Elamipretide in Dry Age-Related Macular Degeneration and Noncentral Geographic Atrophy: ReCLAIM NCGA Study
Abstract
Purpose: Assess the safety, tolerability, and feasibility of subcutaneous administration of the mitochondrialtargeted drug elamipretide in patients with dry age-related macular degeneration (AMD) and noncentral geographic atrophy (NCGA) and to perform exploratory analyses of change in visual function. Design: Phase 1, single-center, open-label, 24-week clinical trial with preplanned NCGA cohort.
Participants: Adults 55 years of age with dry AMD and NCGA.
Methods: Participants received subcutaneous elamipretide 40-mg daily; safety and tolerability assessed throughout. Ocular assessments included normal-luminance best-corrected visual acuity (BCVA), low-luminance BCVA (LLBCVA), normal-luminance binocular reading acuity (NLBRA), low-luminance binocular reading acuity (LLBRA), spectral-domain OCT, fundus autofluorescence (FAF), and patient self-reported function by lowluminance questionnaire (LLQ).
Main Outcome Measures: Primary end point was safety and tolerability. Prespecified exploratory endpoints included changes in BCVA, LLBCVA, NLBRA, LLBRA, geographic atrophy (GA) area, and LLQ.
Results: Subcutaneous elamipretide was highly feasible. All participants (n ¼ 19) experienced 1 or more nonocular adverse events (AEs), but all AEs were either mild (73.7%) or moderate (26.3%); no serious AEs were noted. Two participants exited the study because of AEs (conversion to neovascular AMD, n ¼ 1; intolerable injection site reaction, n ¼ 1), 1 participant discontinued because of self-perceived lack of efficacy, and 1 participant chose not to continue with study visits. Among participants completing the study (n ¼ 15), mean standard deviation (SD) change in BCVA from baseline to week 24 was þ4.6 (5.1) letters (P ¼ 0.0032), while mean change (SD) in LLBCVA was þ5.4 7.9 letters (P ¼ 0.0245). Although minimal change in NLBRA occurred, mean SD change in LLBCVA was e0.52 0.75 logarithm of the minimum angle of resolution units (P ¼ 0.005). Mean SD change in GA area (square root transformation) from baseline to week 24 was 0.14 0.08 mm by FAF and 0.13 0.14 mm by OCT. Improvement was observed in LLQ for dim light reading and general dim light vision.
Conclusions: Elamipretide seems to be well tolerated without serious AEs in patients with dry AMD and NCGA. Exploratory analyses demonstrated possible positive effect on visual function, particularly under low luminance. A Phase 2b trial is underway to evaluate elamipretide further in dry AMD and NCGA.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9560640/pdf/main.pdf
Thompson WR, et al. A phase 2/3 randomized clinical trial followed by an open-label extension to evaluate the effectiveness of elamipretide in Barth syndrome, a genetic disorder of mitochondrial cardiolipin metabolism. Genetics Med. 2021:23:471-78.
Abstract
Purpose: To evaluate effectiveness of elamipretide in Barth syndrome (BTHS), a genetic condition of defects in TAZ, which causes abnormal cardiolipin on the inner mitochondrial membrane.
Methods: We performed a randomized, double-blind, placebo-controlled crossover trial followed by an open-label extension in BTHS to test the effect of elamipretide, a mitochondrial tetrapeptide that interacts with cardiolipin. In part 1, 12 subjects were randomized to 40 mg per day of elamipretide or placebo for 12 weeks, followed by a 4-week washout and then 12 weeks on the opposite arm. Ten subjects continued on the open-label extension (part 2) of 40 mg per day of elamipretide, with eight subjects reaching 36 weeks. Primary endpoints were improvement on the 6-minute walk test (6MWT) and improvement on a BTHS Symptom Assessment (BTHS-SA) scale.
Results: In part 1 neither primary endpoint was met. At 36 weeks in part 2, there were significant improvements in 6MWT (+95.9 m, p = 0.024) and BTHS-SA (-2.1 points, p = 0.031). There were also significant improvements in secondary endpoints including knee extensor strength, patient global impression of symptoms, and some cardiac parameters.
Conclusion: In this interventional clinical trial in BTHS, daily administration of elamipretide led to improvement in BTHS symptoms.
Level 3
Karaa A, et al. Efficacy and Safety of Elamipretide in Individuals With Primary Mitochondrial Myopathy. Neurology. 2023;101(3):e238-e252.
Abstract
Primary mitochondrial myopathies (PMMs) encompass a group of genetic disorders that impair mitochondrial oxidative phosphorylation, adversely affecting physical function, exercise capacity, and quality of life (QoL). Current PMM standards of care address symptoms, with limited clinical impact, constituting a significant therapeutic unmet need. We present data from MMPOWER-3, a pivotal, phase-3, randomized, double-blind, placebo-controlled clinical trial that evaluated the efficacy and safety of elamipretide in participants with genetically confirmed PMM.
Methods: After screening, eligible participants were randomized 1:1 to receive either 24 weeks of elamipretide at a dose of 40 mg/d or placebo subcutaneously. Primary efficacy endpoints included change from baseline to week 24 on the distance walked on the 6-minute walk test (6MWT) and total fatigue on the Primary Mitochondrial Myopathy Symptom Assessment (PMMSA). Secondary endpoints included most bothersome symptom score on the PMMSA, NeuroQoL Fatigue Short-Form scores, and the patient global impression and clinician global impression of PMM symptoms.
Results: Participants (N = 218) were randomized (n = 109 elamipretide; n = 109 placebo). The m0ean age was 45.6 years (64% women; 94% White). Most of the participants (n = 162 [74%]) had mitochondrial DNA (mtDNA) alteration, with the remainder having nuclear DNA (nDNA) defects. At screening, the most frequent bothersome PMM symptom on the PMMSA was tiredness during activities (28.9%). At baseline, the mean distance walked on the 6MWT was 336.7 ± 81.2 meters, the mean score for total fatigue on the PMMSA was 10.6 ± 2.5, and the mean T score for the Neuro-QoL Fatigue Short-Form was 54.7 ± 7.5. The study did not meet its primary endpoints assessing changes in the 6MWT and PMMSA total fatigue score (TFS). Between the participants receiving elamipretide and those receiving placebo, the difference in the least squares mean (SE) from baseline to week 24 on distance walked on the 6MWT was −3.2 (95% CI −18.7 to 12.3; p = 0.69) meters, and on the PMMSA, the total fatigue score was −0.07 (95% CI −0.10 to 0.26; p = 0.37). Elamipretide treatment was well-tolerated with most adverse events being mild to moderate in severity.
Discussion: Subcutaneous elamipretide treatment did not improve outcomes in the 6MWT and PMMSA TFS in patients with PMM. However, this phase-3 study demonstrated that subcutaneous elamipretide is well-tolerated.
https://www.neurology.org/doi/pdf/10.1212/WNL.0000000000207402
Level 4
Escribano-Lopez I, et al. The mitochondrial antioxidant SS-31 increases SIRT1 levels and ameliorates inflammation, oxidative stress and leukocyte-endothelium interactions in type 2 diabetes. Sci Reports. 2018;8:15862.
Abstract
There is growing focus on mitochondrial impairment and cardiovascular diseases (CVD) in type 2 diabetes (T2D), and the development of novel therapeutic strategies in this context. It is unknown whether mitochondrial-targeting antioxidants such as SS-31 protect sufciently against oxidative damage in diabetes. We aimed to evaluate if SS-31 modulates SIRT1 levels and ameliorates leukocyteendothelium interactions, oxidative stress and infammation in T2D patients. Anthropometric and metabolic parameters were studied in 51 T2D patients and 57 controls. Production of mitochondrial reactive oxygen species (ROS), mitochondrial membrane potential, glutathione content, leukocyteendothelium interactions, NFκB-p65, TNFα and SIRT1 levels was measured in leukocytes treated or not with SS-31. We observed increased mitochondrial ROS production that was restored by SS-31 treatment. SS-31 also increased mitochondrial membrane potential, glutathione content, SIRT1 levels and leukocyte rolling velocity and reduced rolling fux and adhesion in T2D patients. NFκB-p65 and TNFα, which were enhanced in diabetic patients, were also reduced by SS-31 treatment. Our results reveal that SS-31 exerts benefcial efects on the leukocytes of T2D patients by reducing oxidative stress, leukocyte-endothelium interactions, NFκB and TNFα and by increasing SIRT1 levels. These actions support its use as a potential agent against CVD risk.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6203778/pdf/41598_2018_Article_34251.pdf
Level 5
Koenig MK, et al. Use of Elamipretide in patients assigned treatment in the compassionate use program: Case series in pediatric patients with rare orphan diseases. JIMD Reports. 2023;64:65-70.
Abstract
Several mitochondrial diseases are caused by pathogenic variants that impair membrane phospholipid remodeling, with no FDA-approved therapies. Elamipretide targets the inner mitochondrial membrane where it binds to cardiolipin, resulting in improved membrane stability, cellular respiration, and ATP production. In clinical trials, elamipretide produced clinical and functional improvements in adults and adolescents with mitochondrial disorders, such as primary mitochondrial myopathy and Barth syndrome; however, experience in younger patients is limited and to our knowledge, these are the first case reports on the safety and efficacy of elamipretide treatment in children under 12 years of age. We describe the use of elamipretide in patients with mitochondrial disorders to provide dosing parameters in patients aged.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9830009/pdf/JMD2-64-65.pdf
Level 6
Pharoah G, et al. The mitochondrially targeted peptide elamipretide (SS-31) improves ADP sensitivity in aged mitochondria by increasing uptake through the adenine nucleotide translocator (ANT). Geroscience. 2023;45(6):3529-48.
Abstract
Aging muscle experiences functional decline in part mediated by impaired mitochondrial ADP sensitivity. Elamipretide (ELAM) rapidly improves physiological and mitochondrial function in aging and binds directly to the mitochondrial ADP transporter ANT. We hypothesized that ELAM improves ADP sensitivity in aging leading to rescued physiological function. We measured the response to ADP stimulation in young and old muscle mitochondria with ELAM treatment, in vivo heart and muscle function, and compared protein abundance, phosphorylation, and S-glutathionylation of ADP/ATP pathway proteins. ELAM treatment increased ADP sensitivity in old muscle mitochondria by increasing uptake of ADP through the ANT and rescued muscle force and heart systolic function. Protein abundance in the ADP/ATP transport and synthesis pathway was unchanged, but ELAM treatment decreased protein s-glutathionylation incuding of ANT. Mitochondrial ADP sensitivity is rapidly modifiable. This research supports the hypothesis that ELAM improves ANT function in aging and links mitochondrial ADP sensitivity to physiological function. ELAM binds directly to ANT and ATP synthase and ELAM treatment improves ADP sensitivity, increases ATP production, and improves physiological function in old muscles. ADP (adenosine diphosphate), ATP (adenosine triphosphate), VDAC (voltage-dependent anion channel), ANT (adenine nucleotide translocator), H+ (proton), ROS (reactive oxygen species), NADH (nicotinamide adenine dinucleotide), FADH2 (flavin adenine dinucleotide), O2 (oxygen), ELAM (elamipretide), -SH (free thiol), -SSG (glutathionylated protein).
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC10643647/pdf/11357_2023_Article_861.pdf
Chen M, et al. Mitochondria-Targeted Peptide MTP-131 Alleviates Mitochondrial Dysfunction and Oxidative Damage in Human Trabecular Meshwork Cells. Glaucoma. 2011;52:7027-37.
Abstract
Purpose.: To investigate the antioxidative ability of a novel mitochondria-targeted peptide MTP-131 in immortalized human trabecular meshwork (iHTM) and glaucomatous human trabecular meshwork (GTM3) cell lines.
Methods.: Cultured iHTM and GTM3 cells were pretreated with MTP-131 for 1 hour, and sustained oxidative stress was induced by subjecting TM cells to 200 μM hydrogen peroxide (H2O2) for 24 hours. Untreated cells and cells incubated with H2O2 alone were used as controls. Lactate dehydrogenase (LDH) assay was used to determine cell viability. Changes of mitochondrial membrane potential (ΔΨm) and generation of intracellular reactive oxygen species (ROS) were analyzed by flow cytometry and confocal microscopy. Activation of caspase 3 was quantified by Western blotting, and apoptosis was measured by flow cytometry. Release of cytochrome c and changes in cytoskeleton were analyzed by confocal microscopy. Data were analyzed with commercial data analysis software and P < 0.05 was considered to be statistically significant.
Results.: In both iHTM and GTM3 cells, decrease of ΔΨm and elevation of intracellular ROS were detected after sustained oxidative stress induced by H2O2. When cells were pretreated with MTP-131, the H2O2-induced mitochondrial depolarization was prevented; intracellular ROS, LDH release, and apoptosis were significantly decreased; release of cytochrome c from mitochondria to cytoplasm and activation of caspase 3 were inhibited. In addition, cytoskeleton changes caused by H2O2 were also alleviated by MTP-131.
Conclusions.: Mitochondria-targeted peptide MTP-131 could prevent both iHTM and GTM3 cells from sustained oxidative stress induced by H2O2.
https://iovs.arvojournals.org/article.aspx?articleid=2165256
Level 7
Campbell MD, et al. Improving mitochondrial function with SS-31 reverses age-related redox stress and improves exercise tolerance in aged mice. Free Radic Biol med. 2019;134:268-81.
Abstract
Sarcopenia and exercise intolerance are major contributors to reduced quality of life in the elderly for which there are few effective treatments. We tested whether enhancing mitochondrial function and reducing mitochondrial oxidant production with SS-31 (elamipretide) could restore redox balance and improve skeletal muscle function in aged mice. Young (5 mo) and aged (26 mo) female C57BL/6Nia mice were treated for 8-weeks with 3 mg/kg/day sS-31. Mitochondrial function was assessed in vivo using 31P and optical spectroscopy. SS-31 reversed age-related decline in maximum mitochondrial ATP production (ATPmax) and coupling of oxidative phosphorylation (P/O). Despite the increased in vivo mitochondrial capacity, mitochondrial protein expression was either unchanged or reduced in the treated aged mice and respiration in permeabilized gastrocnemius (GAS) fibers was not different between the aged and aged+SS-31 mice. Treatment with SS-31 also restored redox homeostasis in the aged skeletal muscle. The glutathione redox status was more reduced and thiol redox proteomics indicated a robust reversal of cysteine S-glutathionylation post-translational modifications across the skeletal muscle proteome. The gastrocnemius in the age+SS-31 mice was more fatigue resistant with significantly greater mass compared to aged controls. This contributed to a significant increase in treadmill endurance compared to both pretreatment and untreated control values. These results demonstrate that the shift of redox homeostasis due to mitochondrial oxidant production in aged muscle is a key factor in energetic defects and exercise intolerance. Treatment with SS-31 restores redox homeostasis, improves mitochondrial quality, and increases exercise tolerance without an increase in mitochondrial content. Since elamipretide is currently in clinical trials these results indicate it may have direct translational value for improving exercise tolerance and quality of life in the elderly.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6588449/
Sabbah HN, et al. Chronic Therapy with Elamipretide (MTP-131), a Novel Mitochondria-Targeting Peptide, Improves Left Ventricular and Mitochondrial Function in Dogs with Advanced Heart Failure. Circ Heart Fail. 2016;9(2):e002206.
Abstract
Background—Elamipretide (MTP-131), a novel mitochondria-targeting peptide, was shown to reduce infarct size in animals with myocardial infarction and improve renal function in pigs with acute and chronic kidney injury. This study examined the effects of chronic therapy with elamipretide on left ventricular (LV) and mitochondrial (MITO) function in dogs with heart failure (HF).
Methods and Results—14 dogs with microembolization-induced HF were randomized to 3 months monotherapy with subcutaneous injections of elamipretide (0.5 mg/kg once daily, HF +ELA, n=7) or saline (Control, HF-CON, n=7). LV ejection fraction (EF), plasma n-terminal probrain natriuretic peptide (nt-pro BNP), tumor necrosis factor-alpha (TNF-α) and C-reactive protein (CRP) were measured before (pre-treatment) and 3 months after initiating therapy (posttreatment). MITO respiration, membrane potential (Δψm), maximum rate of ATP synthesis and ATP/ADP ratio were measured in isolated LV cardiomyocytes obtained at post-treatment. In HFCON dogs, EF decreased at post-treatment compared to pre-treatment (29±1% vs. 31±2%); whereas in HF+ELA dogs, EF significantly increased at post-treatment compared to pre-treatment (36±2% vs. 30±2%, p<0.001).
Conclusions—Long-term therapy with elamipretide improves LV systolic function, normalizes plasma biomarkers and reverses MITO abnormalities in LV myocardium of dogs with advanced HF. The results support the development of elamipretide for the treatment of HF.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4743543/pdf/nihms752478.pdf
Dai W, et al. Bendavia, a mitochondria-targeting peptide, improves postinfarction cardiac function, prevents adverse left ventricular remodeling, and restores mitochondria-related gene expression in rats. J Cardiovasc Pharmacol. 2014;64(6):543-53.
Abstract
AB We evaluated the post-myocardial infarction (MI) therapeutic effects of Bendavia. Two hours after coronary artery ligation, rats were randomized to receive chronic Bendavia treatment (n = 28) or water (n = 26). Six weeks later, Bendavia significantly reduced scar circumference (39.7% +/- 2.2%) compared with water treatment (47.4% +/- 0.03%, P = 0.024) and reduced left ventricular (LV) volume by 8.9% (P = 0.019). LV fractional shortening was significantly improved by Bendavia (28.8% +/- 1.7%) compared with water treatment (23.8% +/- 1.8%, P = 0.047). LV ejection fraction was higher with Bendavia (55.3% +/- 1.4%) than water treatment (49.3% +/- 1.4%, P = 0.005). Apoptosis, within the MI border zone, was significantly less in the Bendavia group (32% +/- 3%, n = 12) compared with the water group (41% +/- 2%, n = 12; P = 0.029). Bendavia reversed mitochondrial function-related gene expression in the MI border, which was largely reduced in water-treated rats. Bendavia improved complex-I and -IV activity, and reduced production of reactive oxygen species and cytosolic cytochrome c level in the peri-infarcted region. Bendavia improved post-MI cardiac function, prevented infarct expansion and adverse LV remodeling, and restored mitochondria-related gene expression, complex-I and -IV activity, and reduced reactive oxygen species and cardiomyocyte apoptosis in the noninfarcted MI border.
No Fulltext Available
Wu J, et al. BDNF pathway is involved in the protective effects of SS-31 on isoflurane-induced cognitive deficits in aging mice. Behav Brain Res. 2016;305:115-21.
Abstract
Mitochondrial dysfunction has been linked to the earliest pathogenesis of isoflurane-induced cognitive impairments in developing or aging mammalian brain. However, its molecular mechanism is poorly understood and a pharmacologic treatment to rapidly reverse mitochondrial dysfunction is lacking. Fifteen-month-old male C57BL/6 mice were exposed to isoflurane for two hours following intraperitoneal administration of mitochondrion-targeted peptide SS-31 or vehicle with 30min interval. The hippocampus was immediately removed for biochemical assays and mitochondria isolation after inhalation. Behavioral tests were evaluated by the open field test and fear conditioning test 24h after the experiment. We showed that cognitive deficits induced by exposure of the aging mice to isoflurane were accompanied by mitochondrial dysfunction in hippocampus due to loss of the enzymatic activity of complex I. This loss resulted in the increase of reactive oxygen species production, decrease of ATP production and mitochondrial membrane potential, and opening of mitochondrial permeability transition pore. Further, we provided evidence that the BDNF signaling pathway was involved in this process to regulate synaptic plasticity-related proteins, for instance, downregulation of synapsin 1, PSD-95 and p-CREB, and upregulation of NR2A, NR2B, CaMKIIα and CaMKIIβ. Of note, the isoflurane-induced cognitive deficits were rescued by SS-31 through reversal of mitochondrial dysfunction, which facilitated the regulation of BDNF signaling including the expression reversal of aforementioned important synaptic-signaling proteins in aging mice. Our data demonstrate that reversing mitochondrial dysfunction by SS-31 enhances BDNF signaling pathway and synaptic plasticity, and provides protective effects on cognitive function, thereby support the notion that SS-31 may have therapeutic benefits for elderly humans undertaking anesthesia.
No Full Text Available
Wu X, et al. Mitochondria-targeted antioxidant peptide SS-31 mediates neuroprotection in a rat experimental glaucoma model. Acta Biochem Biophys Sin (Shanghai). 2019;51(4):411-21.
Abstract
To investigate the neuroprotective effects of the mitochondria-targeted antioxidant Szeto-Schiller peptide 31 (SS-31) in a rat experimental glaucoma model, SS-31 was intraperitoneally (IP) injected into Sprague-Dawley rats, followed by intracameral injection of polystyrene microspheres to induce elevated intraocular pressure (IOP). After 6 weeks, electroretinography (ERG) and flash visual-evoked potentials (F-VEPs) were recorded to assess retinal function. Hematoxylin-eosin staining was performed on retinal cross-sections to measure ganglion cell complex (GCC) thickness. Apoptotic retinal cells were assessed by TUNEL staining. Brn3a-positive retinal ganglion cells (RGCs) were counted in retinal flat mounts via immunofluorescence. The retinal total SOD, SOD2, and MDA expression levels were assessed in retinal tissue homogenates. The cyt c, Bax, and Bcl-2 protein levels in rat retinas were detected by western blot analysis. Bax and Bcl-2 expressions were also evaluated using immunohistochemistry in paraffinized sections. Our results showed that the rats that received microsphere injection developed elevated IOP. SS-31 ameliorated the reductions in the a- and b-wave amplitudes on ERG and the F-VEP amplitude in glaucomatous eyes. GCC thickness was preserved, TUNEL-positive cells were decreased in the retina, and Brn3a-positive RGCs were increased in the SS-31-treated glaucoma group compared with those in the non-treated glaucoma group. SS-31 significantly reduced MDA levels and increased SOD2 levels after glaucoma induction. Significant suppression of cyt c release, upregulation of Bcl-2, and downregulation of Bax were observed following SS-31 administration. In summary, SS-31 exerts neuroprotective effects in this experimental glaucoma model by inhibiting mitochondrial dysfunction and therefore represents a promising therapeutic agent for glaucoma.
https://dds.sciengine.com/cfs/files/pdfs/view/1672-9145/76F3E4EE56384554BD338159EB8C1409.pdf
Kloner RA, et al. Reduction of ischemia/reperfusion injury with bendavia, a mitochondria-targeting cytoprotective Peptide.
Abstract
Background—-Manifestations of reperfusion injury include myocyte death leading to infarction, contractile dysfunction, and vascular injury characterized by the “no-reflow” phenomenon. Mitochondria-produced reactive oxygen species are believed to be centrally involved in each of these aspects of reperfusion injury, although currently no therapies reduce reperfusion injury by targeting mitochondria specifically. Methods and Results—-We investigated the cardioprotective effects of a mitochondria-targeted peptide, Bendavia (Stealth Peptides), across a spectrum of experimental cardiac ischemia/reperfusion models. Postischemic administration of Bendavia reduced infarct size in an in vivo sheep model by 15% (P=0.02) and in an ex vivo guinea pig model by 38% to 42% (P<0.05). In an in vivo rabbit model, the extent of coronary no‐reflow was assessed with Thioflavin S staining and was significantly smaller in the Bendavia group for any given ischemic risk area than in the control group (P=0.0085). Myocardial uptake of Bendavia was ≈25% per minute, and uptake remained consistent throughout reperfusion. Postischemic recovery of cardiac hemodynamics was not influenced by Bendavia in any of the models studied. Isolated myocytes exposed to hypoxia/reoxygenation showed improved survival when treated with Bendavia. This protection appeared to be mediated by lowered reactive oxygen species–mediated cell death during reoxygenation, associated with sustainment of mitochondrial membrane potential in Bendavia‐treated myocytes.
Conclusions: Postischemic administration of Bendavia protected against reperfusion injury in several distinct models of injury. These data suggest that Bendavia is a mitochondria‐targeted therapy that reduces reperfusion injury by maintaining mitochondrial energetics and suppressing cellular reactive oxygen species levels.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3487333/pdf/jah3-1-e001644.pdf
Birk AV, et al. The mitochondrial-targeted compound SS-31 re-energizes ischemic mitochondria by interacting with cardiolipin. J Am Soc Nephrol. 2013;24(8):1250-61.
Abstract
Ischemia causes AKI as a result of ATP depletion, and rapid recovery of ATP on reperfusion is important to minimize tissue damage. ATP recovery is often delayed, however, because ischemia destroys the mitochondrial cristae membranes required for mitochondrial ATP synthesis. The mitochondria-targeted compound SS-31 accelerates ATP recovery after ischemia and reduces AKI, but its mechanism of action remains unclear. Here, we used a polarity-sensitive fluorescent analog of SS-31 to demonstrate that SS-31 binds with high affinity to cardiolipin, an anionic phospholipid expressed on the inner mitochondrial membrane that is required for cristae formation. In addition, the SS-31/cardiolipin complex inhibited cytochrome c peroxidase activity, which catalyzes cardiolipin peroxidation and results in mitochondrial damage during ischemia, by protecting its heme iron. Pretreatment of rats with SS-31 protected cristae membranes during renal ischemia and prevented mitochondrial swelling. Prompt recovery of ATP on reperfusion led to rapid repair of ATP-dependent processes, such as restoration of the actin cytoskeleton and cell polarity. Rapid recovery of ATP also inhibited apoptosis, protected tubular barrier function, and mitigated renal dysfunction. In conclusion, SS-31, which is currently in clinical trials for ischemia-reperfusion injury, protects mitochondrial cristae by interacting with cardiolipin on the inner mitochondrial membrane.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3736700/
Bai J, et al. SS-31 protect retinal pigment epithelial cells from H2O2-induced cell injury by reducing apoptosis. Clin Exp Pharmacol Physiol. 2021;48(7):941-1040.
Abstract
Evidence has shown that effects from oxidative stress induced damage of retinal or human retinal pigment epithelial (RPE) cells. Antioxidant supplementation is a plausible strategy to avoid oxidative stress and maintain the function of retina. d-Arg2,6-dimethyltyrosine-Lys-Phe-NH2 (SS-31) has been used in the treatment of many diseases. In this study, we found that SS-31 attenuated hydrogen peroxide (H2O2)- induced loss of cell viability, reduced oxidative damage and cell apoptosis in RPE cells. HO-1, Trx-1 and Nrf-2 expression levels significantly increased on pre-treatment with SS-31 compared with the H2O2 group. SS-31 inhibited apoptosis through the downregulation of Bax and the upregulation of Bcl-2. Our results suggest that SS-31 had a protective effect against H2O2 treatment in ARPE-19 cells by enhancing the antioxidative enzymes expression and decreasing apoptosis, which could be considered a promising therapeutic intervention for retinal degeneration.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8252508/pdf/CEP-48-1016.pdf
Level 8
Szeto HH. First-in-class cardiolipin-protective compound as a therapeutic agent to restore mitochondrial bioenergetics. Br J Pharmacol. 2014;171(8):2029-50.
Abstract
A decline in energy is common in aging, and the restoration of mitochondrial bioenergetics may offer a common approach for the treatment of numerous age-associated diseases. Cardiolipin is a unique phospholipid that is exclusively expressed on the inner mitochondrial membrane where it plays an important structural role in cristae formation and the organization of the respiratory complexes into supercomplexes for optimal oxidative phosphorylation. The interaction between cardiolipin and cytochrome c determines whether cytochrome c acts as an electron carrier or peroxidase. Cardiolipin peroxidation and depletion have been reported in a variety of pathological conditions associated with energy deficiency, and cardiolipin has been identified as a target for drug development. This review focuses on the discovery and development of the first cardiolipin-protective compound as a therapeutic agent. SS-31 is a member of the Szeto-Schiller (SS) peptides known to selectively target the inner mitochondrial membrane. SS-31 binds selectively to cardiolipin via electrostatic and hydrophobic interactions. By interacting with cardiolipin, SS-31 prevents cardiolipin from converting cytochrome c into a peroxidase while protecting its electron carrying function. As a result, SS-31 protects the structure of mitochondrial cristae and promotes oxidative phosphorylation. SS-31 represents a new class of compounds that can recharge the cellular powerhouse and restore bioenergetics. Extensive animal studies have shown that targeting such a fundamental mechanism can benefit highly complex diseases that share a common pathogenesis of bioenergetics failure. This review summarizes the mechanisms of action and therapeutic potential of SS-31 and provides an update of its clinical development programme.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3976620/pdf/bph0171-2029.pdf
Zhu Y, et al. SS-31, a mitochondria-targeted peptide, ameliorates kidney disease. Oxid Med Cell Longev. 2022;2022;1295509.
Abstract
Mitochondria are essential for eukaryotic cell activity and function, and their dysfunction is associated with the development and progression of renal diseases. In recent years, there has been a rapid development in mitochondria-targeting pharmacological strategies as mitochondrial biogenesis, morphology, and function, as well as dynamic changes in mitochondria, have been studied in disease states. Mitochondria-targeting drugs include nicotinamide mononucleotide, which supplements the NAD+ pool; mitochondria-targeted protective compounds, such as MitoQ; the antioxidant coenzyme, Q10; and cyclosporin A, an inhibitor of the mitochondrial permeability transition pore. However, traditional drugs targeting mitochondria have limited clinical applications due to their inability to be effectively absorbed by mitochondria in vivo and their high toxicity. Recently, SS-31, a mitochondria-targeting antioxidant, has received significant research attention as it decreases mitochondrial reactive oxygen species production and prevents mitochondrial depolarization, mitochondrial permeability transition pore formation, and Ca2+-induced mitochondrial swelling, and has no effects on normal mitochondria. At present, few studies have evaluated the effects of SS-31 against renal diseases, and the mechanism underlying its action is unclear. In this review, we first discuss the pharmacokinetics of SS-31 and the possible mechanisms underlying its protective effects against renal diseases. Then, we analyze its renal disease-improving effects in various experimental models, including animal and cell models, and summarize the clinical evidence of its benefits in renal disease treatment. Finally, the potential mechanism underlying the action of SS-31 against renal diseases is explored to lay a foundation for future preclinical studies and for the evaluation of its clinical applications.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9192202/pdf/OMCL2022-1295509.pdf
Szeto HH. Cardiolipin-targeted peptides rejuvenate mitochondrial function, remodel mitochondria, and promote tissue regeneration during aging. Acrh Biochem Biophys. 2018;660:137-48.
Abstract
It has been proposed that a loss of bioenergetic capacity of cells contributes to the progressive loss of biological function with age. Aging is associated with loss of mitochondrial cristae membranes and inhibition of ATP production. Despite the many approaches being pursued for improving mitochondrial function, none of them directly targets the electron transport chain to improve ATP production. Recent studies have brought attention to cardiolipin as a unique target for promoting mitochondrial efficiency. Cardiolipin is important for cristae curvatures and is necessary for optimal activity of the respiratory complexes and the assembly of supercomplexes. Here we describe the discovery of a class of cell-penetrating aromatic-cationic tetrapeptides that selectively target cardiolipin and increase coupling efficiency while reducing reactive oxygen species production. These compounds can rejuvenate mitochondrial bioenergetics, remodel mitochondrial cristae structure, repair cellular structure, and restore organ function during aging.
No Full Text Available
[i] Birk AV, et al. The mitochondrial-targeted compound SS-31 re-energizes ischemic mitochondria by interacting with cardiolipin. J Am Soc Nephrol. 2013;24(8):1250-61.
[ii] Szeto HH. First-in-class cardiolipin-protective compound as a therapeutic agent to restore mitochondrial bioenergetics. Br J Pharmacol. 2014;171(8):2029-50.
[iii] Wu J, et al. BDNF pathway is involved in the protective effects of SS-31 on isoflurane-induced cognitive deficits in aging mice. Behav Brain Res. 2016;305:115-21.
[iv] Zhu Y, et al. SS-31, a mitochondria-targeted peptide, ameliorates kidney disease. Oxid Med Cell Longev. 2022;2022;1295509.
[v] Zhu Y, et al. SS-31, a mitochondria-targeted peptide, ameliorates kidney disease. Oxid Med Cell Longev. 2022;2022;1295509.
[vi] Bai J, et al. SS-31 protect retinal pigment epithelial cells from H2O2-induced cell injury by reducing apoptosis. Clin Exp Pharmacol Physiol. 2021;48(7):941-1040.
[vii] Wu X, et al. Mitochondria-targeted antioxidant peptide SS-31 mediates neuroprotection in a rat experimental glaucoma model. Acta Biochim Biophys Sin (Shanghai). 2019;51(4):411-21.
[viii] Chen M, et al. Protective effect of mitochondria-targeted peptide MTP-131 against oxidative stress-induced apoptosis in RGC-5 cells. Mol Med Rep. 2017;15(4):2179-85.
[ix] Wu X, et al. Mitochondria-targeted antioxidant peptide SS-31 mediates neuroprotection in a rat experimental glaucoma model. Acta Biochim Biophys Sin (Shanghai). 2019;51(4):411-21.
[x] Escribano-Lopez I, et al. The mitochondrial antioxidant SS-31 increases SIRT1 levels and ameliorates inflammation, oxidative stress and leukocyte-endothelium interactions in type 2 diabetes. Sci Reports. 2018;8:15862.
[xi] Escribano-Lopez I, et al. The mitochondrial antioxidant SS-31 increases SIRT1 levels and ameliorates inflammation, oxidative stress and leukocyte-endothelium interactions in type 2 diabetes. Sci Reports. 2018;8:15862.
[xii] Szeto HH. First-in-class cardiolipin-protective compound as a therapeutic agent to restore mitochondrial bioenergetics. Br J Pharmacol. 2014;171(8):2029-50.
[xiii] Szeto HH. First-in-class cardiolipin-protective compound as a therapeutic agent to restore mitochondrial bioenergetics. Br J Pharmacol. 2014;171(8):2029-50.
[xiv] Zhao W, et al. Elamipretide (SS-31) improves mitochondrial dysfunction, synaptic and memory impairment induced by lipopolysaccharide in mice. J Neuroinflam. 2019;16:230.
[xv] Zhao W, et al. Elamipretide (SS-31) improves mitochondrial dysfunction, synaptic and memory impairment induced by lipopolysaccharide in mice. J Neuroinflam. 2019;16:230.
[xvi] Szeto HH. Cardiolipin-targeted peptides rejuvenate mitochondrial function, remodel mitochondria, and promote tissue regeneration during aging. Acrh Biochem Biophys. 2018;660:137-48.
[xvii] Campbell MD, et al. Improving mitochondrial function with SS-31 reverses age-related redox stress and improves exercise tolerance in aged mice. Free Radic Biol med. 2019;134:268-81.