TP508
TP-508
TP-508 acetate
TP508 acetate
TRAP-508
Chrysalin®
Rusalatide acetate
Thrombin Peptide
Contents
Most Frequent Uses:
- Musculoskeletal injuries
- Wounds including diabetic foot ulcers – topically
- Chronic inflammatory conditions including:
- SARS-Cov2 infection
- Acute lung injury – Acute Respiratory Distress Syndrome (ARDS)
- Chronic smoke inhalation
- Musculoskeletal injuries
- Radiation damage – radioprotective, including GUT crypt cells
- Vascular damage – including myocardial ischemia
Dosage:
- SubQ – Locally for injury/wound
- For injury healing – 10 – 30 mcg SubQ at site of injury x1 single dose
- Must be given w/in 24 hrs of injury
- SubQ – for aesthetics
- 15 units to site
- Give 4 times, 1 month apart then repeat annually
- Topically
- 10 mcg TP508 in small amount saline, apply to area then cover wound with foam bandage
- Use this twice weekly along with routine wound care.
Safety and Potential Side Effects/Contraindications:
- TP508 peptides administered subcutaneously and applied topically are reported safe in recommended dosages.
- As with all injections, redness, and pain at the site of injection may be present.
Description
TP508 is a 23 amino acid synthetic peptide representing a portion of human thrombin.[i] TP508 stimulates angiogenic responses in animal models of dermal wound healing, in chick chorioallantoic membranes, and in cultured human aortic and microvascular endothelial cells.[ii],[iii] TP508 has been reported to initiate tissue repair and regeneration by reversing endothelial dysfunction, stimulating revascularization, attenuating inflammation, stimulating NO production, and reducing apoptosis. TP508 is also reported to protect crypt cells in the GUT exposed to radiation by significantly increasing expression of both LGR5 and DCLK1 stem cell markers in intestinal crypts as well as the number of DCLK1-positive cells per crypt.[iv]
The positive effects of TP508 involve changes in the inflammatory response, enhancing cell recruitment and angiogenesis.[v]
Early studies reported that thrombin, the serine protease responsible for fibrin clot formation, initiated cell proliferation and other cellular postclotting events through a growth factor-like mechanism that involved its binding and activation of specific thrombin receptors on the surface of fibroblasts and other cells.[vi] Although many of the cellular effects of thrombin appear to require proteolytic activity and activation of proteolytically activated receptors, studies report that binding of thrombin or thrombin derivatives without proteolytic activity promotes a number of cellular events involved in tissue repair and wound healing.[vii] These observations have led to the hypothesis that nonproteolytic peptide fragments of thrombin released from a fibrin clot during early stages of wound repair may modulate inflammation and promote healing.
Unlike thrombin, which is activated at the site of injury, TP508 peptide has no enzymatic activity and does not promote or interfere with blood coagulation.[viii] Pre-clinical safety studies have reported that the peptide can be injected intravenously or intraperitonealy at doses of up to 25 mg/kg with no adverse effects, that it is classified as a nonsensitizer based on hamster skin sensitivity testing, and that topical treatment of open porcine wounds (followed upon wound closure with dermal injection at the wound site) of 100 mg/day for 20 weeks had no apparent negative effects (OrthoLogic Corp., unpublished results).
“When injected into the fracture, ChrysalinÒ (TP508), a novel synthetic peptide, is thought to speed the process of bony healing and reduce the time that patients are required to wear casts or bulky fixation devices,” said Dr. Scott W. Wolfe, Attending Orthopedic Surgeon and Chief of the Hand Service at HSS (Hospital for Special Surgery, Manhattan NYC).
In human clinical trials, TP508 was reported to significantly increase healing of diabetic foot ulcers and distal radius fractures with no drug-related adverse events.[ix] Systemic administration of TP508 enhances VEGF-stimulated angiogenesis and attenuates effects of chronic hypoxia.1
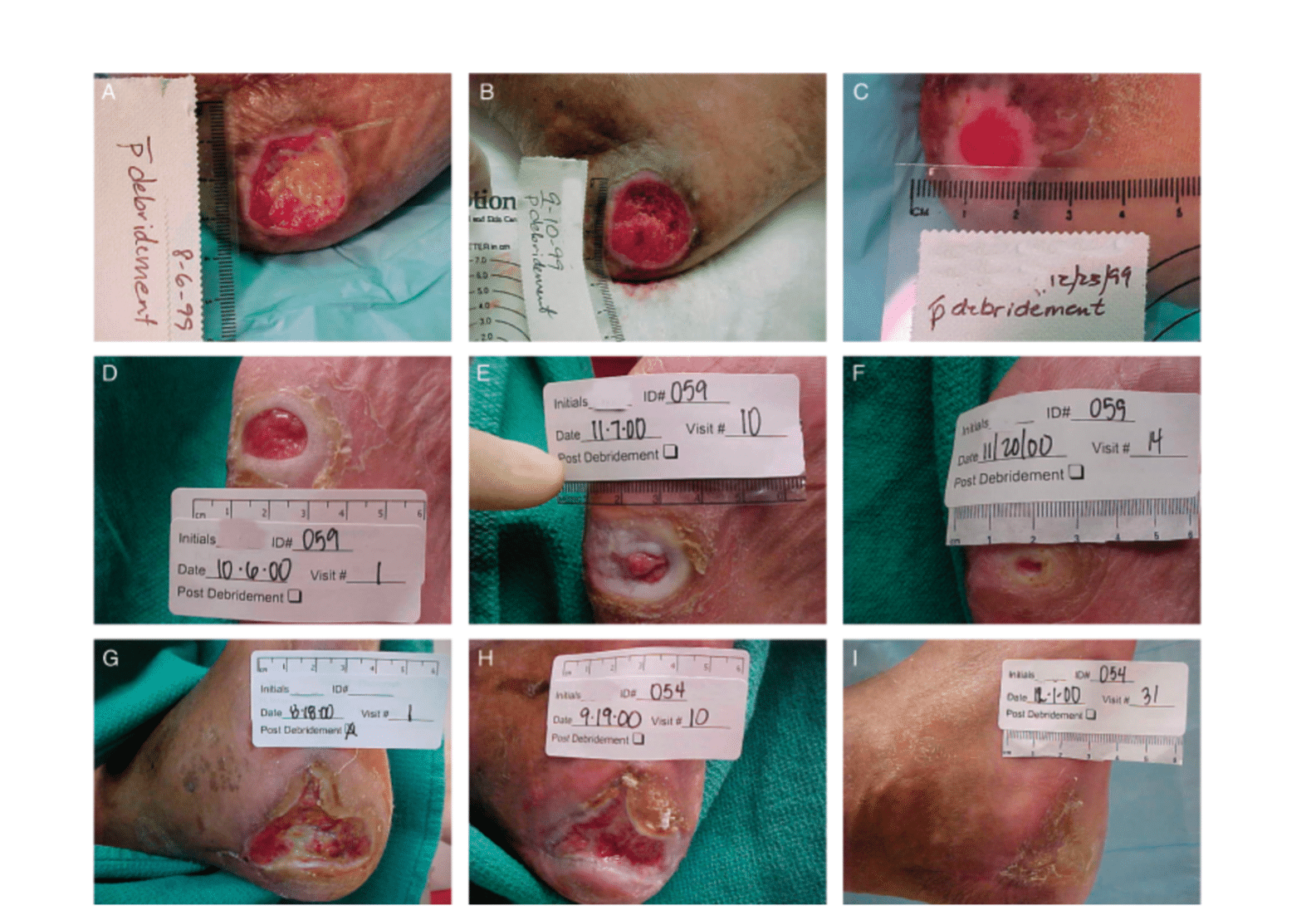
Effect of Chrysalin on diabetic foot ulcer on the heel of the foot.
- Slides A,B,C are placebo w/ debridement for 2 months;
- Slides D,E,F are treated w/ 1 mcg Chrysalin topically 2x week x 2 months w/ debridement;
- Slides G,H,I are treated w/ 10 mcg Chrysalin topically 2x week x 2 months w/ debridement
Data from: Fife C, et al. Wound Repair Regen. 2007;15(1):23-34.
Chrysalin Specifics
ChrysalinÒ (TP508) is an “investigational drug” that is currently being evaluated under Food and Drug Administration’s investigational new drug application for diabetic foot ulcers (phase 1/2) and fracture repair (phase 3) by OrthoLogic Corp (Tempe, AZ). Preclinical studies showed that TP508 accelerates wound healing in both incisional and normal dermal full-thickness wounds[x],[xi] and in dermal tissue made ischemic by creation of surgical flaps.[xii] TP508 also stimulates healing of rat fractures,[xiii] segmental bone defects,[xiv],[xv] and distraction osteogenesis defects.[xvi] In all of these animal models, TP508 stimulates early revascularization.[xvii],[xviii] Moreover, the angiogenic potential of TP508 in the absence of other factors present in vivo was confirmed by in vitro studies using endothelial cells and vessel sprouting from microvascular segments.[xix],[xx]
Clinical Research
IPS Level of Evidence
IPS Clinical Pharmacists have developed a method of ranking the studies so that the practitioner can easily discern the level of evidence this study provides to the topic. Levels 1-8 are listed below:
| Level of Evidence | Description | |
| X | Level 1 | FDA Approved Drug studies |
| Level 2 | Evidence obtained from systematic review and/or meta-analyses of studies including RCTs and other human studies | |
| Level 3 | Evidence obtained from a RCT | |
| X | Level 4 | Evidence obtained from a study without randomization |
| Level 5 | Evidence obtained from case reports | |
| X | Level 6 | Evidence obtained from in vitro human studies |
| X | Level 7 | Evidence obtained from laboratory animal studies |
| X | Level 8 | Evidence obtained from Opinions or Reviews |
Levels
Level 1
Fife C, et al. Thrombin peptide Chrysalin stimulates healing of diabetic foot ulcers in a placebo-controlled phase I/II study. Wound Repair Regen. 2007;15(1):23-34.
Abstract
Thrombin and thrombin peptides play a role in initiating tissue repair. The potential safety and efficacy of TP508 (Chrysalin) treatment of diabetic foot ulcers was evaluated in a 60-subject, prospective, randomized, double-blind, placebo-controlled phase I/II clinical trial. Chrysalin in saline or saline alone was applied topically, twice weekly, to diabetic ulcers with standardized care and offloading. A dose-dependent effect was seen in the per-protocol population where 1 and 10 mug Chrysalin treatment resulted in 45 and 72% more subjects with complete healing than placebo treatment. Chrysalin treatment of foot ulcers more than doubled the incidence of complete healing (p<0.05), increased mean closure rate approximately 80% (p<0.05), and decreased the median time to 100% closure by approximately 40% (p<0.05). Chrysalin treatment of heel ulcers within this population resulted in mean closure rates 165% higher than placebos (p<0.02) and complete healing in 86% (6/7) of ulcers compared with 0% (0/5) of placebo ulcers (p<0.03). Local wound reactions and adverse events (AEs) were equal between groups with no reported drug-related changes in laboratory tests or serious AEs. These results indicate the potential safety and efficacy of Chrysalin for treatment of diabetic foot ulcers.
https://www.experts.com/content/articles/stone-article-chrysalin_study_whs_2007.pdf
Level 6
Li G,et al. rhBMP-2, rhVEGF(165), rhPTN and thrombin-related peptide, TP508 induce chemotaxis of human osteoblasts and microvascular endothelial cells. J Orthop Res. 2005;23: 680-685.
Abstract
Osteogenesis and angiogenesis are inter-linked and tightly regulated processes involved in growth, repair, and bone remodeling. Bone morphogenic protein 2 (BMP-2), vascular endothelial growth factor (VEGF), pleiotrophin (PTN) and thrombin-related peptide, TP508 have all been found to have the ability to promote bone fracture healing by enhancing both the osteogenesis and angiogenesis processes. One of the underlying mechanisms proposed is that mediators for osteogenesis may also be involved in mediating angiogenesis and vice versa. The aim of this study was to examine the chemotactic effects of rhBMP-2, rhVEGF(165), rhPTN and TP508 on human osteoblasts and endothelial cells. Using a direct-viewing chemotaxis assay system, we report for the first time, the direct quantitative observation of chemotaxis of both human osteoblastc cells and microvascular endothelial cells towards sources of rhBMP-2, rhVEGF(165), rhPTN and TP508. This study confirmed that rhBMP-2, rhVEGF(165), rhPTN and TP508 have chemotactic effects on both human osteoblastic and endothelial cells, indicating that these factors are directly involved in promoting angiogenesis and osteogenesis by recruiting osteoblasts and endothelial cells via chemotaxis.
Vartanian KB, et al. The non- proteolytically active thrombin peptide TP508 stimulates angiogenic sprouting. J Cell Physiol. 2006;206:175-180.
Abstract
Thrombin is a serine protease that promotes platelet aggregation, blood coagulation, and tissue repair. A peptide derived from a non-proteolytically active region of thrombin, TP508, also promotes tissue repair and increased vascularity, yet does not activate platelet and inflammatory cascades. TP508 binds to cells with high affinity and stimulates cells independent of the proteolytically active thrombin receptors (PARs) and thus is considered to activate a non-proteolytically active receptor (non-PAR) pathway. Using a model of angiogenic sprouting, we further defined the angiogenic potential of TP508 and investigated the role of non-proteolytic, thrombin-mediated pathways in angiogenesis. The assay involves measuring angiogenic sprouting from cultured, intact microvessel fragments. In this assay, TP508 stimulated angiogenic sprouting to an extent similar to or greater than the potent angiogenic factor, VEGF. However, TP508 had no significant effect on the number of sprouts that formed per vessel. In contrast to TP508, proteolytically active receptor agonists had no effect or inhibited angiogenic sprouting. The increased sprouting activity stimulated by TP508 was VEGF dependent but did not involve an increase in VEGF mRNA expression above baseline levels. These results suggest that TP508 acts early in angiogenesis and directly on microvascular cells to accelerate sprouting, but not to induce more sprouting, in a manner different than the intact thrombin molecule.
No Full Text Available
Freyberg S, et al. Thrombin Peptide (TP508) Promotes Adipose Tissue-Derived Stem Cell Proliferation via PI3 Kinase/Akt Pathway. J Vasc Res. 2009;46(2):98-102.
Abstract
A synthetic peptide representing the receptor-binding domain of human thrombin (TP508) promotes angiogenesis and accelerates wound healing in animal models. However, the mechanisms underlying the therapeutic effects of TP508 have not been clearly defined. In this study, we set out to determine whether TP508 could stimulate stem cell proliferation. Adipose tissue-derived stem cells (ASCs) were incubated with TP508 (5 μg/ml) and cell proliferation was determined by bromodeoxyuridine (BrdU) incorporation. Our data showed that TP508 treatment significantly stimulated BrdU incorporation in ASCs (p < 0.01). The increased BrdU incorporation induced by TP508 was abolished by the PI3 kinase (PI3K) inhibitor LY294002 at 50 μM. Western blot analysis of ASCs revealed increased phosphorylation of Akt in response to TP508 when compared to unstimulated controls. These results indicate that TP508 exerts proliferative effects on ASCs via the PI3K/Akt pathway.
https://web.archive.org/web/20160821231216id_/http://www.karger.com:80/Article/PDF/142727
Naldini A, et al. The thrombin peptide, TP508, enhances cytokine release and activates signaling events. Peptides 2004;25:1917–1926.
Abstract
The thrombin peptide, TP508, accelerates tissue repair and initiates a cascade of cellular events. We have previously shown that alpha-thrombin induces cytokine expression in human mononuclear cells. We, therefore, investigated the possibility that TP508 might activate cytokine production and intracellular signaling pathways associated with cytokine activation. Our results show that TP508 induces cytokine expression in human mononuclear cells. TP508 treatment enhances extracellular signal-regulated kinase (Erk1/2) activities in U937 cells, as well as Erk1/2 and p38 activation in Jurkat T cells. These data support the hypothesis that TP508 may accelerate tissue repair through the activation of the inflammatory response.
No Full Text Available
Level 7
Kantara C, et al. Novel regenerative peptide TP508 mitigates radiation-induced gastrointestinal damage by activating stem cells and preserving crypt integrity. Lab Invest. 2015;95:1222-33.
Abstract
In recent years, increasing threats of radiation exposure and nuclear disasters have become a significant concern for the United States and countries worldwide. Exposure to high doses of radiation triggers a number of potentially lethal effects. Among the most severe is the gastrointestinal (GI) toxicity syndrome caused by the destruction of the intestinal barrier, resulting in bacterial translocation, systemic bacteremia, sepsis and death. The lack of effective radioprotective agents capable of mitigating radiation-induced damage has prompted a search for novel countermeasures that can mitigate the effects of radiation post-exposure, accelerate tissue repair in radiation-exposed individuals, and prevent mortality. We report that a single injection of regenerative peptide TP508 (rusalatide acetate, Chrysalin®) 24h after lethal radiation exposure (9Gy, LD100/15) appears to significantly increase survival and delay mortality by mitigating radiation-induced intestinal and colonic toxicity. TP508 treatment post-exposure prevents the disintegration of gastrointestinal crypts, stimulates the expression of adherens junction protein Ecadherin, activates crypt cell proliferation, and decreases apoptosis. TP508 post-exposure treatment also up-regulates the expression of DCLK1 and LGR5 markers of stem cells that have been shown to be responsible for maintaining and regenerating intestinal crypts. Thus, TP508 appears to mitigate the effects of GI toxicity by activating radioresistant stem cells and increasing the stemness potential of crypts to maintain and restore intestinal integrity. These results suggest that TP508 may be an effective emergency nuclear countermeasure that could be delivered within 24h post-exposure to increase survival and delay mortality, giving victims time to reach clinical sites for advanced medical treatment.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4626368/pdf/nihms-706507.pdf
Norfleet AM, et al. Thrombin peptide, TP508, stimulates angiogenic responses in animal models of dermal wound healing, in chick chorioallantoic membranes, and in cultured human aortic and microvascular endothelial cells. Gen Pharmacol. 2000;35:249-254.
Abstract
The thrombin peptide, TP508, also known as Chrysalin (OrthoLogic, Tempe, Arizona), is a twenty-three-amino-acid peptide that represents a portion of the receptor-binding domain of the native human thrombin molecule that has been identified as the binding site for a specific class of receptors on fibroblasts and other cells. Preclinical studies with this peptide have shown that it can accelerate tissue repair in both soft and hard tissues by mechanisms that appear to involve up-regulation of genes that initiate a cascade of healing events. These events include recruitment and activation of inflammatory cells, directed migration of cells (chemotaxis), cell proliferation, elaboration of extra-cellular matrix, and accelerated revascularization of the healing tissues. Early preclinical dermal wound-healing studies showed that TP508 accelerated healing of both incisional wounds and full-thickness excisional wounds in normal and ischemic skin. In all of these studies, the accelerated healing was associated with increased neovascularization across the incision or in the granulating wound bed. Studies in a rat fracture model have also shown that TP508 accelerates the rate of fracture repair. Gene array analysis of fracture callus from control and TP508-treated fractures indicated that TP508 treatment was associated with an up-regulation of early response elements, inflammatory mediators, and genes related to angiogenesis. Similar to what had been seen in dermal wounds, histology from rat fracture callus twenty-one days after treatment indicated that fractures treated with TP508 had significantly more large functional blood vessels than did fractures in the control animals. In vitro studies support these in vivo data and indicate that TP508 may have a direct angiogenic effect by promoting the rate of new vessel growth. The results from phase-1 and phase-2 human clinical studies have shown a positive stimulatory effect of TP508 in the healing of diabetic ulcers and in the repair of fractures to the distal aspect of the radius. Collectively, these studies suggest that TP508 accelerates tissue repair by initiating a cascade of events that lead to an increased rate of tissue revascularization and regeneration.
No Full Text Available
Fossum TW, et al. TP508 (Chrysalin®®) Reverses EndothelialDysfunction and Increases Perfusion andMyocardial Function in Hearts With Chronic Ischemia. 214-225.
Abstract
Endothelial dysfunction (ED) is characterized by impaired nitric oxide (NO) signaling, decreased NO-dependent vasodilatation, increased vascular inflammation, and diminished response to angiogenic factors. TP508 (Chrysalin®), an angiogenic tissue repair peptide, was tested for potential effects on myocardial revascularization and ED using a porcine model of chronic myocardial ischemia. TP508 increased perfusion in ischemic regions up to16-fold (P < .02) and doubled myocardial wall thickening (P < .02) relative to placebo controls. Ischemic arterioles exhibited impaired NO-mediated vasodilation and diminished NO production. TP508 reversed ischemic effects, increasing NO-mediated vasodilation (P < .05), endothelial nitric oxide synthase (eNOS) expression, and NO production. In human endothelial cells, TP508 stimulated eNOS activation (1.84 ± 0.2-fold; P < .02), increased NO production (85 ± 18%; P < .02), and prevented hypoxia-induced eNOS downregulation (P < .01). Thus, TP508 reverses ED both in porcine ischemic hearts and cultured human endothelial cells. These results suggest potential therapeutic benefit of TP508 in myocardial revascularization and treatment of ED-related diseases.
https://journals.sagepub.com/doi/epdf/10.1177/1074248408321468
Sheller MR, et al. Repair of rabbit segmental defects with the thrombin peptide, TP508. J Orthop Res. 2004;22:1094-1099.
Abstract
The synthetic peptide, TP508 (Chrysalin), was delivered to rabbit segmental bone defects in biodegradable controlled-release PLGA microspheres to determine its potential efficacy for enhancing healing of non-critically and critically sized segmental defects. Non-critically sized radial defects were created in the forelimbs of New Zealand White rabbits, which were randomized into three treatment groups receiving 10, 50 and 100 microg doses of TP508 in the right radius and control microspheres (without TP508) in the left radius. Torsional testing of the radii at six weeks showed a significant increase in ultimate torque, failure torque, ultimate energy, failure energy, and stiffness when treated with TP508 compared to controls (p<0.01 for all measures). Thus, TP508 appeared to enhance or accelerate bone growth in these defects. In a second set of experiments, critically sized ulnar defects were created in the forelimbs of New Zealand White rabbits, which were randomized into two groups with each rabbit receiving microspheres with 100 or 200 microg of TP508 into the right ulnar defect and control microspheres (without TP508) alone into the left ulnar defect. Bone healing was evaluated with plain radiographs, synchrotron-based microtomography, and mechanical testing. Radiographs of the rabbit limbs scored by three blinded, independent reviewers demonstrated a significantly higher degree of healing when treated with TP508 than their untreated control limbs (p<0.05). Three-dimensional synchrotron tomography of a limited number of samples showed that the new bone in TP508-treated samples had a less porous surface appearance and open marrow spaces, suggesting progression of bone remodeling. Torsional testing of the ulnae at nine weeks showed a significant increase in maximum torque and failure energy when treated with TP508 compared to controls (p<0.01 for both measures). These results suggest that TP508 in a controlled release delivery vehicle has the potential to enhance healing of segmental defects in both critically and non-critically sized defects.
https://onlinelibrary.wiley.com/doi/pdfdirect/10.1016/j.orthres.2004.03.009
Wang H, Li X, Tomin E, et al. Thrombin peptide (TP508) promotes fracture repair by up-regulating inflammatory mediators, early growth factors, and increasing angiogenesis. J Orthop Res. 2005;23:671-679.
Abstract
Previous studies have shown that a single injection of thrombin peptide (TP508) accelerates fracture repair in a closed rat femoral fracture model. The present study was conducted to elucidate the molecular mechanisms of TP508 action using Affymetrix genomescale profiling and to link early gene expression changes to fracture histology and bone strength changes. Treatment of femoral fractures with TP508 accelerated fracture repair as determined by destructive torsion testing. Blinded histological analysis demonstrated that TP508-treated fracture callus had a significant increase in blood vessels relative to the controls. Gene array analysis showed that TP508 significantly induced expression of early growth factors, inflammatory response modifiers, and angiogenesis-related genes. This study therefore suggests that TP508 promotes fracture repair through a mechanism that involves an increased induction of a number of growth factors, enhanced expression of inflammatory mediators, and angiogenesis-related genes.
https://onlinelibrary.wiley.com/doi/pdf/10.1016/j.orthres.2004.10.002
StiernbergJ, et al. Acceleration of full-thickness wound healing in normal rats by the synthetic thrombin peptide, TP508. Wound Repair Regen. 2000;8:204-215.
Abstract
Thrombin is an essential factor in hemostasis, inflammation, and tissue repair. The synthetic thrombin peptide, TP508, binds to high-affinity thrombin receptors and mimics cellular effects of thrombin at sites of tissue injury. Treatment of full-thickness excisional wounds in normal rats with a single topical application of 0.1 microg TP508 (14 pmol/cm2) reproducibly accelerates wound closure, yielding wounds that on average close 39% more than controls by day 7 (p < 0.001). Wounds treated with 1.0 microg TP508 are 35% and 43% (p < 0.001) smaller than controls on day 7 and 10, respectively. The early rate of closure is approximately 40% greater in TP508-treated than vehicle-treated wounds (20 versus 14 mm2/day) and remains higher through day 7. Breaking strength after closure is slightly greater (15-23%) in wounds treated with TP508 than with saline alone. Histologic comparisons show that TP508 enhances recruitment of inflammatory cells to the wound site within 24 hours post-injury. TP508 treatment also augments revascularization of injured tissue, as evidenced at day 7 by the larger size of functional vessels in the granulation tissue and by the directed development of blood vessels to wounds. These studies raise the possibility that TP508 may be clinically useful in management of open wounds.
No Full Text Available
Li G, et al. Bone formation is enhanced by thrombin-related peptide TP508 during distraction osteogenesis. J Orthop Res. 2005;23:196-202.
Abstract
The thrombin-related peptide, TP508, has been shown to promote soft tissue healing and fracture repair. One possible clinical application of TP508 is to accelerate bone regeneration during distraction osteogenesis, which is a lengthy procedure involving significant complications. In this study, we tested the ability of TP508 to accelerate the consolidation phase of distraction osteogenesis in a rabbit model of leg lengthening. Twenty-three rabbits had left tibiae lengthened for 1 cm over a period of 6 days. TP 508 (0, 30 and 300 microg in 300 microl saline) was injected into the distraction gaps at the beginning and the end of the lengthening phase, and all the animals were killed 2 weeks after lengthening. By the end of experiment, more animals in the TP508 treated groups had complete bony union of the distraction gaps when compared to the saline treated group. pQCT examination of the regenerates demonstrated a significantly greater bone mineral density (BMD) in the TP508 treated groups relative to the saline control group, but no statistical difference in the BMD was found between the two dosages of TP508. Bone consolidation and bone remodeling was far advanced in the TP508 300 microg treated group, and the regenerates mainly consisted of well-vascularized woven bone. In contrast, in the group that received the 30 microg TP508 treatment, focal bone defects and discontinuities of the new cortices were evident in some but not all animals. In the saline control group a majority of the animals showed large amounts of fibrous and cartilaginous tissues in the regenerates, and none of the regenerates had completed consolidation. This study has demonstrated that local application of TP508 enhanced bone formation and consolidation during distraction osteogenesis in the rabbit. The findings indicate that TP508 may be useful in promoting osteogenesis in situations when augmentative treatment for bone formation and consolidation are needed.
Level 8
Olszewska-Pazdrak B, et al. Thrombin Peptide TP508 Stimulates Rapid Nitric Oxide Production in Human Endothelial Cells. J Vascular Res. 2010;47(3):203-13.
Abstract
TP508, a 23-amino-acid peptide representing a portion of human thrombin, promotes tissue revascularization and repair. The molecular mechanisms of TP508 action, however, remain unclear. Nitric oxide (NO) plays a crucial role in regulation of angiogenesis and wound healing. We, therefore, investigated TP508 effects on NO production in human endothelial cells. TP508 stimulated a rapid, dose-dependent, 2- to 4-fold increase in NO production. TP508 induced NO release as early as 5 min. Continued exposure to TP508 for 1–24 h increased NO concentrations over controls by 100.5 ± 9.6 and 463.3 ± 24.2 nM, respectively. These levels of NO release were similar to those produced in response to vascular endothelial growth factor (VEGF). TP508- and VEGF-induced NO production was decreased by inhibitors of PI-3K (LY294002) and Src (PP2). TP508 stimulated early transient phosphorylation of Src and Akt. In contrast to VEGF, TP508 stimulation of NO release was inhibited by PKC inhibitor (Go6976) and was independent of intracellular calcium mobilization. These results demonstrate that TP508 and VEGF stimulate NO production to similar levels but through distinct pathways. This study provides new insights into the initial molecular mechanisms by which TP508 may stimulate diverse cellular effects leading to tissue revascularization and wound healing.
Ryaby JT, et al. Thrombin peptide TP508 stimulates cellular events leading to angiogenesis, revascularization, and repair of dermal and musculoskeletal tissues. J Bone Joint Surg Am. 2006;88(suppl 3):132-139.
Abstract
The thrombin peptide, TP508, also known as Chrysalin (OrthoLogic, Tempe, Arizona), is a twenty-three-amino-acid peptide that represents a portion of the receptor-binding domain of the native human thrombin molecule that has been identified as the binding site for a specific class of receptors on fibroblasts and other cells. Preclinical studies with this peptide have shown that it can accelerate tissue repair in both soft and hard tissues by mechanisms that appear to involve up-regulation of genes that initiate a cascade of healing events. These events include recruitment and activation of inflammatory cells, directed migration of cells (chemotaxis), cell proliferation, elaboration of extra-cellular matrix, and accelerated revascularization of the healing tissues. Early preclinical dermal wound-healing studies showed that TP508 accelerated healing of both incisional wounds and full-thickness excisional wounds in normal and ischemic skin. In all of these studies, the accelerated healing was associated with increased neovascularization across the incision or in the granulating wound bed. Studies in a rat fracture model have also shown that TP508 accelerates the rate of fracture repair. Gene array analysis of fracture callus from control and TP508-treated fractures indicated that TP508 treatment was associated with an up-regulation of early response elements, inflammatory mediators, and genes related to angiogenesis. Similar to what had been seen in dermal wounds, histology from rat fracture callus twenty-one days after treatment indicated that fractures treated with TP508 had significantly more large functional blood vessels than did fractures in the control animals. In vitro studies support these in vivo data and indicate that TP508 may have a direct angiogenic effect by promoting the rate of new vessel growth. The results from phase-1 and phase-2 human clinical studies have shown a positive stimulatory effect of TP508 in the healing of diabetic ulcers and in the repair of fractures to the distal aspect of the radius. Collectively, these studies suggest that TP508 accelerates tissue repair by initiating a cascade of events that lead to an increased rate of tissue revascularization and regeneration.
Full Text Not Available
[i] Glenn KC, Frost GH, Bergmann JS, Carney DH. Synthetic peptides bind to high-affinity thrombin receptors and modulate thrombin mitogenesis. Pept Res. 1988;1:65-73.
[ii] Ryaby JT, et al. Thrombin peptide TP508 stimulates cellular events leading to angiogenesis, revascularization, and repair of dermal and musculoskeletal tissues. J Bone Joint Surg Am. 2006;88(suppl 3):132-139.
[iii] Norfleet AM, Bergmann JS, Carney DH. Thrombin peptide, TP508, stimulates angiogenic responses in animal models of dermal wound healing, in chick chorioallantoic membranes, and in cultured human aortic and microvascular endothelial cells. Gen Pharmacol. 2000;35:249-254.
[iv] Kantara C, et al. Novel regenerative peptide TP508 mitigates radiation-induced gastrointestinal damage by activating stem cells and preserving crypt integrity. Lab Invest. 2015;95:1222-33.
[v] Naldini A, et al. The thrombin peptide, TP508, enhances cytokine release and activates signaling events. Peptides. 2004;25:1917–1926.
[vi] Weisel JW, Litvinov RI. Fibrin formation, structure and properties. Subcell Biochem. 2017;82:405-56.
[vii] Naldini A, Carraro F, Baldari CT et al. The thrombin peptide, TP508, enhances cytokine release and activates signaling events. Peptides 2004;25:1917–1926.
[viii] Olszewska-Pazdrak B, et al. Thrombin Peptide TP508 Stimulates Rapid Nitric Oxide Production in Human Endothelial Cells. J Vascular Res. 2010;47(3):203-13.
[ix] Fife C, et al. Thrombin peptide Chrysalin stimulates healing of diabetic foot ulcers in a placebo-controlled phase I/II study. Wound Repair Regen. 2007;15(1):23-34.
[x] Carney DH, et al. Enhancement of incisional wound healing and neovascularization in normal rats by thrombin and synthetic thrombin receptor- activating peptides. J Clin Invest. 1992;89:1469-1477.
[xi] StiernbergJ, et al. Acceleration of full-thickness wound healing in normal rats by the synthetic thrombin peptide, TP508. Wound Repair Regen. 2000;8:204-215.
[xii] Norfleet AM, et al. Thrombin peptide TP508 accelerates closure of dermal excisions in animal tissue with surgically induced ischemia. Wound Repair Regen. 2000;8:517-529.
[xiii] Wang H, Li X, Tomin E, et al. Thrombin peptide (TP508) promotes fracture repair by up-regulating inflammatory mediators, early growth factors, and increasing angiogenesis. J Orthop Res. 2005;23:671-679.
[xiv] Sheller MR, Crowther RS, Kinney JH, et al. Repair of rabbit segmental defects with the thrombin peptide, TP508. J Orthop Res. 2004;22:1094-1099.
[xv] Hedberg EL, Kroese-Deutman HC, Shih CK, et al. Effect of varied release kinetics of the osteogenic throm- bin peptide TP508 from biodegradable polymeric scaffolds on bone formation in vivo. J Biomed Mater Res A. 2005;72:343-353.
[xvi] Li G, Ryaby JT, Carney DH, Wang H. Bone formation is enhanced by thrombin-related peptide TP508 during distraction osteogenesis. J Orthop Res. 2005;23:196-202.
[xvii] Norfleet AM, Bergmann JS, Carney DH. Thrombin peptide, TP508, stimulates angiogenic responses in animal models of dermal wound healing, in chick chorioallantoic membranes, and in cultured human aortic and microvascular endothelial cells. Gen Pharmacol. 2000;35:249-254.
[xviii] Ryaby JT, et al. Thrombin peptide TP508 stimulates cellular events leading to angiogenesis, revascularization, and repair of dermal and musculoskeletal tissues. J Bone Joint Surg Am. 2006;88(suppl 3):132-139.
[xix] Vartanian KB, Chen HY, Kennedy J, et al. The non- proteolytically active thrombin peptide TP508 stimulates angiogenic sprouting. J Cell Physiol. 2006;206:175-180.
[xx] Li G, Cui Y, McIlmurray L, Allen WE, Wang H. rhBMP-2, rhVEGF(165), rhPTN and thrombin-related peptide, TP508 induce chemotaxis of human osteoblasts and microvascular endothelial cells. J Orthop Res. 2005;23: 680-685.