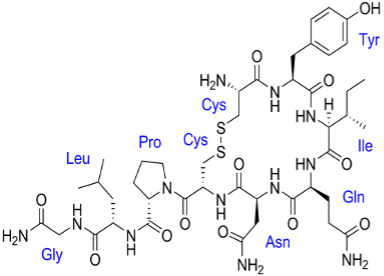
Oxytocin
Intranasal Spray
Sublingual Tabs
Contents
Most Frequent Uses:
- Intranasal spray and Sublingual Tab
- Improved social functioning
- Improved sexual arousal, orgasm and sexual performance in men and women
- Improved sexual lubrication in women
- Pain control support
- Prader-Willi syndrome
- Psychiatric Issues
- Anxiety
- Addiction
- Phobias – improved trust and behavioral inhibition
- Autism Spectrum Disorder
- Schizophrenia
- PTSD
- Panic disorder
Dosage(s):
General Dosage: Oxytocin Nasal Spray 
- Clinical studies report using a single dose of 24-48 IU or 24-48
IU QD x 2 weeks
- Compounding Pharmacies make multiple concentrations, depending on individual Pharmacy and/or practitioner need.
- Generally available as 5 IU/0.1ml (per spray) to 200 IU/0.1ml (per spray), with many concentrations in between, again depending on individual Pharmacy and/or practitioner need.
General Dosage: Oxytocin ODT (Orally Disintegrating Tab)* 
-
- Generally available as 10-100 IU oxytocin per ODT
- Dissolve 1 ODT sublingually 1/2 hour before sexual activity
- Compounding Pharmacies make multiple concentrations, depending on individual Pharmacy and/or practitioner need.
* Some compounding pharmacies will add other components to oxytocin ODT, such as tadalafil or sildenafil in differing amounts (ex: from 10-125 IU oxytocin + 50-60mg sildenafil) – for synergistic effects. If this is the case, drug interactions and SE’s associated with PDE5 inhibitors should be considered.
Safety and Potential Side Effects/Contraindications:
- Intranasal and sublingual oxytocin is reported safe and efficacious in recommended dosages.
- Do NOT use in pregnancy – Oxytocin is indicated during pregnancy to induce labor; it precipitates uterine contractions and abortion.[i]
- Oxytocin is excreted in the breast milk but is not expected to have adverse effects in the infant (Oxytocin, Drugs and Lactation Database (LactMed), 2023).
- 2015 RCT in 97 healthy participants reported exogenous oxytocin administration had no effect on testosterone, progesterone, or cortisol in women or men.[ii]
- Oxytocin may possess antidiuretic effects, and prolonged use can increase the possibility of an antidiuretic effect.[iii] Use caution when using oxytocin in those with hypertension and renal issues.[iv]
- Common adverse events associated with oxytocin nasal spray are:
- Nasal discomfort
- Tiredness
- Irritability
- Diarrhea
- Minor skin irritations
- Uncommon adverse events associated with oxytocin nasal spray are: [v]
- Seizures
- Hyperactivity/aggression
[i] Cates W, Schulz KF. Contraception. 1980;22(5):513-25.
[ii] Wirth MM, et al. Effects of intranasal oxytocin on steroid hormones in men and women. Neurophychobiology. 20125;71:202-11.
[iii] Li C, et al. Molecular mechanisms of antidiuretic effect of oxytocin. J Am Soc Nephrol. 2008;19(2):225-232.
[iv] Phie J, et al. Prolonged Subcutaneous Administration of Oxytocin Accelerates Angiotensin II-Induced Hypertension and Renal Damage in Male Rats. PLoS One. 2015;10(9):e0138048.
[v] Yatawara CJ, et al. The effect of oxytocin nasal spray on social interaction deficits observed in young children with autism: A randomized clinical crossover trial. Mol. Psychiatry 2016; 21: 1225–1231.
Description
Oxytocin was the first peptide hormone to be biochemically described and synthesized. Commonly called the “love hormone, oxytocin is a pleiotropic, cyclic nonapeptide that also acts as a neurotransmitter in the posterior pituitary of the brain, leading to uterine contraction and milk-ejection. Oxytocin is naturally produced when you touch or interact with another person. Together with the neuropeptide vasopressin, it is believed to influence social cognition and behavior. Oxytocin has a role as an oxytocic and a vasodilator agent. Oxytoxin is then introduced into the peripheral circulation via the posterior pituitary gland or released into the central nervous system to act on receptors widely distributed throughout the brain. Oxytocin has broad implications for general health, adaptation, development, reproduction, and social behavior. Endogenous oxytocin and stimulation of the oxytocin receptor support patterns of growth, resilience, and healing. Oxytocin can function as a stress-coping molecule, an anti-inflammatory, and an antioxidant, with protective effects especially in the face of adversity or trauma. Oxytocin influences the autonomic nervous system and the immune system.
Dopamine interacts with oxytocin to regulate the activity of the reward circuitry in the brain.[1] Again, oxytocin is synthesized in organs including the placenta, heart, kidneys, uterus, ovaries, testis, blood vessels, and skin.[2] Oxytocin receptors are present on myoepithelial cells, the pancreas, heart, blood vessels, kidneys, thymus, adipocytes, and macrophages. Oxytocin receptors are involved in nitric oxide production, uterine contractions, and lipolysis through G protein coupling and C-beta pathway activation. The production and secretion of oxytocin in the gastrointestinal tract are associated with autocrine and paracrine effects.[3]
Uses
Oxytocin continues to be an important tool in modern obstetrics to induce labor when indicated and to manage postpartum hemorrhage. It is estimated that labor induction with oxytocin is used in almost 10% of deliveries globally. It should be noted that there are risks associated with oxytocin intervention during childbirth. Oxytocin should be used judiciously only when necessary and by experienced healthcare practitioners. Although most commonly linked to labor and delivery, oxytocin actually has broad peripheral and central effects. It plays an important role in pair bonding, social cognition and functioning, and even fear conditioning. Oxytocin also serves a role in metabolic homeostasis and cardiovascular regulation.
More recently, intranasally delivered oxytocin has been reported to stimulate social behaviors, improve sexual performance and exert pain-relieving, helps suppress appetite, helps with psychiatric disorders from anxiety, addiction to schizophrenia and provide anti-stress/anti-inflammatory and restorative effects. Oxytocin has been tested in the treatment of conditions as apparently diverse as autism spectrum disorders, schizophrenia, postpartum depression, anxiety, post-traumatic stress disorders, borderline personality, addiction, pain, metabolic and digestive disorders, diabetes, cardiovascular diseases, cancer, and infectious diseases (to name only a few).[4]
Oxytocin has been tested in the treatment of many conditions, including:[5]
- Autism spectrum disorder
- Schizophrenia
- Postpartum depression
- Anxiety
- Post-traumatic stress disorder
- Borderline personality
- Addiction, including food, drugs and alcohol
- Pain
- Metabolic conditions, such as obesity
- Digestive disorders
- Cardiovascular disease
- Cancer
- Infectious diseases
- Decreased muscle mass and strength
Why Intranasal and Sublingual Oxytocin?
The nasal cavity may offer a noninvasive route for oxytocin delivery to the brain via olfactory and trigeminal nerve transport. After administration of both intranasal and intravenous oxytocin in rodents and humans, effects are seen only after intranasal administration.
Intranasal pharmacokinetics are below:
- Absorption
Administration of oxytocin nasal spray resulted in an increase in oxytocin plasma levels from 20.8 ± 5.9 pg/mL at baseline to 41.1 ± 19.9 pg/mL after 15 minutes and 35.1 ± 16.4 pg/mL after 30 minutes.
- Distribution
Elevated oxytocin has also been reported to be detectable in cerebrospinal fluid and serum in humans after an intranasal administration of 24 IU, indicating that intranasal oxytocin has sufficient bioavailability.[vi] 2013 study looked at intranasal oxytocin and concentrations in cerebrospinal fluid (CSF) and plasma.[vii] The patients received either 24 IU of oxytocin (n = 11) or placebo (n = 4). Results show that oxytocin levels significantly increased in both plasma and CSF. However, whereas oxytocin plasma concentrations peaked at 15 min after intranasal administration and decreased after 75 min, CSF concentrations took up to 75 min to reach a significant level. Moreover, there was no correlation between oxytocin plasma and CSF concentrations.
- Excretion
Oxytocin is rapidly removed from plasma by the liver and kidneys, with only small amounts being excreted unchanged in the urine.
Conventionally, synthetic oxytocin (marketed as Pitocin® and Syntocinon®) is commonly administered intravenously to induce or augment labor in pregnant women. Traditionally, oxytocin has been examined for its role in childbirth, lactation, and maternal attachment behavior.
Given recent advances in delivering oxytocin via nasal administration, oxytocin has seen an exponential growth of research examining its effects on social cognition and behavior. For example, animal research has reported that intranasal administration of oxytocin to pairs of rats increases the duration of physical contact with each other[viii], whereas administration of an oxytocin-receptor antagonist in male rats is associated with reduced social exploration of a con-specific male rat.[ix] In human populations, studies have reported that oxytocin increases trust during a money transfer game[x], improves memory for positive social information[xi] and attenuates amygdala reactivity to emotional stimuli.[xii]
* However, note that a 2021 study reported that oral oxytoxin (as sublingual tabs), but not intranasal oxytocin administration increased both arousal ratings for faces and associated brain reward responses, the latter being partially mediated by blood concentration changes.[xiii] Furthermore, while oral oxytocin increased amygdala and arousal responses to face emotions, after intranasal administration they were decreased. [xiv]
Compounded Oxytocin Uses
The peptide hormone oxytocin plays important roles in mammalian social behavior, including maternal care, pair bonding, and social memory.[xv] Recently, there has been interest in the effects of oxytocin on emotional processing, social cognition, and social behavior in humans. The research is made possible by using a nasal spray which allows a noninvasive experimental manipulation of oxytocin. Increases in cerebrospinal fluid are observed after the nasal administration of oxytocin and related peptides,[xvi],[xvii] and oxytocin accumulation in brain sites relevant to emotion and memory was observed after nasal administration to laboratory animals.[xviii] There are several possible routes by which this peptide could directly or indirectly affect the human brain after nasal administration.[xix] Whether direct or indirect, acute intranasal oxytocin does exert effects on the brain: many effects of oxytocin have been described on behavior, cognition, and physiology, including changes in neural activation in functional magnetic resonance imaging studies
Sexual Dysfunction
Oxytocin has been studied in pre- and postmenopausal women with female sexual dysfunction.[xx],[xxi],[xxii] A 2018 study evaluated oxytocin serum levels before and after sexual intercourse in women affected by anorgasmia.[xxiii] 15 anorgasmic women and 16 orgasmic women had serum oxytocin levels measured before sexual intercourse and 5 min after coital sexual activity. Anorgasmic women had an unpleasant sexual experience and were stressed, whereas orgasmic women were fully satisfied with their sexual activity. Prior to sexual intercourse, anorgasmic women had lower levels of oxytocin than orgasmic women. After sex, oxytocin levels did not change in anorgasmic women. Orgasmic women had higher levels of oxytocin than anorgasmic women, in both instances. The repetitive processes to experience the sexual body sensations could represent a survival behavior of species by attachment to a partner.
In 2014, Behnia evaluated 29 heterosexual couples (n=58) with acute effects of 24IU of intranasally administered oxytocin. Oxytocin was found to increase intensity of orgasm and contentment after sexual intercourse. Women reported feeling more relaxed and subgroups indicated better abilities to share sexual desires or to empathize with their partners.[xxiv]
In a randomized, prospective, double-blind, placebo controlled, crossover trial, Muin et al studied oxytocin in pre- and postmenopausal women (41-65 years old) with sexual dysfunction over a 22-week period.[xxv] Patients were instructed to inhale 32 IU of oxytocin intranasally up to 50 minutes before intercourse. Female Sexual Function Index scores (FSFI) increased by 26%, Sexual Quality of Life (SQOL-F) scores increased 144%, Sexual Interest and Desire (SIDI-F) scores, increased by 29%, and Female Sexual Distress (FSDS) scores decreased by 36%. The authors concluded that long term intranasal oxytocin improves (1) Sexual Function, (2) Symptoms of Depression, and (3) Sexual Satisfaction.
In 2021, Kou et al reported using oxytocin sublingually (oral) administration, but not intranasal oxytocin administration, increased both arousal ratings for faces and associated brain reward responses, the latter being partially mediated by blood concentration changes. Furthermore, while oral oxytocin increased amygdala and arousal responses to face emotions, after intranasal administration they were decreased.[xxvi]
Psychiatric Issues
Studies have reported that oxytocin improves emotion recognition ability in autistic youth, and patients with schizophrenia.[xxvii] A recent meta-analysis reported that intranasal oxytocin may enhance the recognition of facial expressions of emotion.[xxviii] Also, several experiments have investigated the effect of intranasal oxytocin in patients with social anxiety disorder (SAD). One study examined the impact of administering oxytocin as an adjunct to exposure therapy for SAD and found that patients treated with oxytocin showed a positive effect in increasing positive evaluations of appearance and speech performance.[xxix] Another study demonstrated that OT modulates amygdala reactivity in patients with generalized SAD.[xxx] These studies are consistent with previous research in healthy individuals, which report that oxytocin may have therapeutic potential given its anxiolytic and prosocial effects.
One recent small study in 2007 reported a negative correlation between anxiety symptoms and oxytocin in a group of 25 depressed patients.[xxxi] However, other studies have found a positive association between oxytocin and anxiety symptoms; for example, in 29 patients with obsessive compulsive disorder, higher oxytocin was associated with greater anxiety symptoms.[xxxii]
A 2015 a RCT investigated the efficacy of an extended treatment of oxytocin nasal spray combined with social cognition training (SCT) to improve social cognition, clinical symptoms, and social functioning in early psychosis in 52 individuals (aged 16–35 years) diagnosed with an early psychosis schizophrenia-spectrum illness.[xxxiii] Participants received oxytocin (24 International Units) or placebo nasal spray twice-daily for 6 weeks, combined with group SCT (2 × 1 hour weekly sessions for 6 weeks). An additional dose of oxytocin was administered before each weekly session. Results showed that on all primary and secondary outcomes, there was no benefit of oxytocin nasal spray treatment in comparison to placebo. Exploratory post hoc analysis suggested that increased use of nasal spray was, however, associated with reductions in negative symptoms in the oxytocin condition only.
Oxytocin and Autism Spectrum Disorder
Oxytocin has been used as a potential therapy to reduce social impairment in autism spectrum disorder. In animals, oxytocin increases social approach and social memory, both of which are impaired in persons with autism.[xxxiv] Some studies have reported reduced plasma oxytocin levels in children with autism spectrum disorder.[xxxv] A meta-analysis has tentatively supported an association between autism spectrum disorder and polymorphisms in the oxytocin receptor gene, OXTR, but not at the level of genomewide significance.[xxxvi] Elevated promoter methylation in OXTR has also been reported in persons with autism spectrum disorder, as compared with controls.[xxxvii] Decreased oxytocin-receptor density has been found in the ventral pallidum of postmortem brain tissue obtained from a few persons with autism spectrum disorder.[xxxviii]
Several clinical trials have reported that a single dose of intranasal oxytocin enhanced performance on measures of social cognition or motivation, as compared with placebo, in persons with autism spectrum disorder.[xxxix] These results have been supported by functional neuroimaging studies involving persons with autism spectrum disorder, which have reported differences in regional brain activation in response to social stimuli after the administration of intranasal oxytocin, as compared with placebo.[xl],[xli] Small, randomized, controlled trials of intranasal oxytocin administered for 4 to 24 weeks in persons with autism spectrum disorder have had equivocal results with regard to oxytocin-associated improvements in social functioning, social cognition, or social attention.[xlii],[xliii] The inconsistent results among these various investigations may have been the result of limited power or differences in participant age, oxytocin formulation or dose, treatment duration, outcome measures, or analytic methods.[xliv],[xlv]
A 2017 meta-analysis of 80 clinical trials reported intranasal oxytocin as being well tolerated as a possible treatment in the ASD population. The authors recommended larger-scale clinical trials.[xlvi]
Pain
Intranasal oxytocin has gained increasing attention in recent years as a promising analgesic.[xlvii],[xlviii] Oxytocin is a neuropeptide that is produced in the hypothalamus, and released into the peripheral and central nervous system through independent pathways.[xlix] Evidence suggests that oxytocin may be a safe and effective method for pain management.[l],[li] Oxytocin may decrease pain sensitivity through three mechanisms: (1) oxytocin is transported to an area involved in pain modulation, Laminae I, II and IV of the dorsal horn, through a hypothalamic–spinal projection.[lii] Approximately 35% of neurons in the dorsal horn contain oxytocin receptors that act to inhibit pain-carrying Aδ-fibres and C-fibres[liii]; (2) oxytocin binds to opioid receptors, and results in analgesic effects when administered to the periaqueductal grey, and effect that can be blocked with an opioid antagonist.[liv],[lv] Furthermore, analgesic effects of endogenous and exogenous oxytocin can be blocked by the opioid antagonist naloxone[lvi]; and (3) oxytocin may decrease pain sensitivity by improving mood, reducing anxiety and buffering stress given that the induction of negative emotions are associated with heightened pain and autonomic arousal.[lvii],[lviii] In an informative controlled trial, intranasal administration of oxytocin in men resulted in greater calmness, less anxiety and a trend toward lower cortisol during the Trier Social Stress Test.[lix]
Preliminary evidence suggests that oxytocin may be an effective adjuvant analgesic that is applicable to a broad patient population. A systematic review of the effect of oxytocin on pain in animals and humans is available.[lx]
Oxytocin Weight Loss
Oxytocin is a neuropeptide involved in the homeostasis of food consumption and energy and it affects hedonic eating. Studies in obese or binge-eating patients reported the hypophagic effect of oxytocin, which reduced caloric intake after administration.[lxi] Several studies have reported the effect of oxytocin’s increasing energy intake, decreasing food consumption, and contributing to weight loss. Oxytocin’s effects on food intake and metabolism suggest its therapeutic potential for treating obesity and binge eating.
In studies, intranasal treatment with oxytocin reduced the intake of tasty food such as those high in fats and carbohydrates, resulting in weight loss.[lxii],[lxiii] Oxytocin decreased food intake by controlling compensatory hedonic eating.[lxiv] Oxytocin’s inhibition of reward-related food motivation1 was associated with satiety signals and inhibited activity of the food-related reward pathway.[lxv]
A meta-analysis of oxytocin’s effects on feeding in animals by Leslie et al. reported different results according to sex and the oxytocin administration period.[lxvi] Oxytocin decreased food consumption more in male rats than in female rats, with food intake decreasing over time.
The mechanism by which oxytocin reduces food intake has not been established. However, one hypothesized mechanism of the effects of oxytocin on caloric intake involves the action of oxytocin on homeostatic, reward, and impulse control brain circuitry, which could reduce calorie consumption, particularly of more appetizing foods in response to peripheral signals indicating energy availability.[lxvii]
Single-dose administration of oxytocin showed calorie and weight loss effects, and some studies analyzed the results of chronic oxytocin administration. In a study where 24 IU of oxytocin was administered intranasally four times a day for eight weeks, the participants lost an average of 4.6 kg after four weeks and an average of 8.9 kg after eight weeks. More weight was lost by the more obese participants.[lxviii] A meta-analysis reported greater decreases in food consumption because of oxytocin in males compared to females, in the satiated state compared to the fasting state, and in more obese subjects compared to less.[lxix]
Clinical Research
IPS Level of Evidence
IPS Clinical Pharmacists have developed a method of ranking the studies so that the practitioner can easily discern the level of evidence this study provides to the topic. Levels 1-8 are listed below:
| Level of Evidence | Description | |
| Level 1 | FDA Approved Drug studies | |
| X | Level 2 | Evidence obtained from systematic review and/or meta-analyses of studies including RCTs and other human studies |
| X | Level 3 | Evidence obtained from a RCT |
| X | Level 4 | Evidence obtained from a study without randomization |
| Level 5 | Evidence obtained from case reports | |
| X | Level 6 | Evidence obtained from in vitro human studies |
| X | Level 7 | Evidence obtained from laboratory animal studies |
| X | Level 8 | Evidence obtained from Opinions or Reviews |
Levels
Level 2
Wang Y, et al. Oxytocin therapy for core symptoms in autism spectrum disorder: an updated meta-analysis of randomized controlled trials. Res Autism Spectr Disord 2019; 64: 63–75.
Abstract
Purpose: The purpose of this article is to examine the efficacy of oxytocin in treating core symptoms of autism spectrum disorder (ASD) with children.
Methods: A systematic literature search was conducted to identify randomized controlled trials (RCTs) of oxytocin for the treatment of core symptoms in children with ASD. The search included studies published between January 1, 1999 and March 15, 2023, that were randomized, single or double-blinded, and included a placebo control group. Standard screening rules were applied to select relevant studies, resulting in the inclusion of five RCTs involving 486 children with ASD.
Results: Ultimately, a total of five RCTs, involving 486 children with ASD, were included in the review using standard screening rules.One of the included studies demonstrated a statistically significant improvement in Social Responsiveness Scale (SRS) and Repetitive Behavior Scale-Revised (RBS) scores when children with ASD were treated with oxytocin (24 IU/2 days for 6 weeks). The improvement in core symptoms persisted at the 6-month follow-up. The meta-analysis findings suggested that oxytocin might have a moderate effect in improving the core symptom of narrow interests and repetitive stereotyped behaviors in children with ASD.
Conclusion: While the therapeutic value of oxytocin in treating core symptoms of ASD in children is not fully established, the results of this meta-analysis indicate a potential moderate effect. However, further studies with larger sample sizes and more robust RCTs are needed to directly demonstrate the efficacy of oxytocin. Future research should also focus on effect size and outcome evaluation accuracy while minimizing bias in RCT experiments.
No Full Text Available
Keech B, et al. Intranasal oxytocin, social cognition and neuro-developmental disorders: a meta-analysis. Psychoneuroendocrinology 2018; 87: 9–19.
Abstract
Deficits in social cognition are pervasive and characteristic of neurodevelopmental disorders (NDDs). Clinical trials of intranasal oxytocin (IN-OT) to improve social cognition have yielded inconclusive results. The current study is a meta-analysis of randomized controlled trials (RCTs) considering the effect of IN-OT on social cognitive domains across a range of NDDs. Medline, PsychINFO and Scopus were searched for RCTs published through to July 25, 2017. Seventeen studies met inclusion criteria, comprising 466 participants with a NDD. Meta-analysis using a random-effects model, revealed that IN-OT had no significant effect on emotion recognition (Hedges’ g=0.08), a moderate but non-significant effect on empathy (Hedges’ g=0.49), and a small, significant effect on theory of mind (ToM) (Hedges’ g=0.21). Meta-regression indicated that the effect of IN-OT on social cognition was not moderated by the diagnosis or age of participants, or the dose or frequency of IN-OT administration. The results highlight a need for more well-designed RCTs, as it remains difficult to draw conclusions about the potential for IN-OT to improve social cognition in NDDs. The promise of IN-OT should be considered tentative.
No Full Text Available
Cai Q, et al. Systematic review and meta-analysis of reported adverse events of long-term intranasal oxytocin treatment for autism spectrum disorder. Psychiat Clin Neurosci. 2018;72(3):140-51.
Abstract
Recent studies have suggested oxytocin as a possible drug to treat social deficits caused by autism spectrum disorder (ASD), but the safety of intranasal oxytocin in autistic patients has not been established. The aim of this review was to characterize the side-effect profile of long-term intranasal oxytocin in treatment of ASD compared to placebo. All randomized controlled trials of intranasal oxytocin in the treatment of ASD published before 1 January 2017 that reported safety data were identified from databases, including PubMed, Embase, Cochrane Library, and International Pharmaceutical Abstract. Relevant data from the selected studies were then extracted for meta-analysis to estimate the pooled risk ratio for the most common adverse events. Descriptive analysis of severe adverse events was also conducted. Of the 223 participants in the five included studies, 123 were given oxytocin and 100 were given placebos. Nasal discomfort (14.3%), tiredness (7.2%), irritability (9.0%), diarrhea (4.5%), and skin irritation (4.5%) were the most common adverse events. None of these common adverse events was statistically associated with treatment allocation according to meta-analysis using pooled data (all P-values > 0.1). Five severe adverse events were reported, namely aggression (one in placebo, two in oxytocin) and seizures (one in placebo, one in oxytocin). Results from this systematic review support intranasal oxytocin as well tolerated and safe for use in the ASD population. Larger clinical trials should be conducted to establish the efficacy of intranasal oxytocin as a treatment of ASD.
Level 3
Watanabe T, et al. Clinical and neural effects of six-week administration of oxytocin on core symptoms of autism. Brain 2015; 138: 3400–12.
Abstract
Autism spectrum disorder is a prevalent neurodevelopmental disorder with no established pharmacological treatment for its core symptoms. Although previous literature has shown that single-dose administration of oxytocin temporally mitigates autistic social behaviours in experimental settings, it remains in dispute whether such potentially beneficial responses in laboratories can result in clinically positive effects in daily life situations, which are measurable only in long-term observations of individuals with the developmental disorder undergoing continual oxytocin administration. Here, to address this issue, we performed an exploratory, randomized, double-blind, placebo-controlled, crossover trial including 20 high-functional adult males with autism spectrum disorder. Data obtained from 18 participants who completed the trial showed that 6-week intranasal administration of oxytocin significantly reduced autism core symptoms specific to social reciprocity, which was clinically evaluated by Autism Diagnostic Observation Scale (P = 0.034, PFDR < 0.05, Cohen’s d = 0.78). Critically, the improvement of this clinical score was accompanied by oxytocin-induced enhancement of task-independent resting-state functional connectivity between anterior cingulate cortex and dorso-medial prefrontal cortex (rho = -0.60, P = 0.011), which was measured by functional magnetic resonance imaging. Moreover, using the same social-judgement task as used in our previous single-dose oxytocin trial, we confirmed that the current continual administration also significantly mitigated behavioural and neural responses during the task, both of which were originally impaired in autistic individuals (judgement tendency: P = 0.019, d = 0.62; eye-gaze effect: P = 0.03, d = 0.56; anterior cingulate activity: P = 0.00069, d = 0.97; dorso-medial prefrontal activity: P = 0.0014, d = 0.92; all, PFDR < 0.05). Furthermore, despite its longer administration, these effect sizes of the 6-week intervention were not larger than those seen in our previous single-dose intervention. These findings not only provide the evidence for clinically beneficial effects of continual oxytocin administration on the core social symptoms of autism spectrum disorder with suggesting its underlying biological mechanisms, but also highlight the necessity to seek optimal regimens of continual oxytocin treatment in future studies.
https://academic.oup.com/brain/article/138/11/3400/331027?login=false
Yatawara CJ, et al. The effect of oxytocin nasal spray on social interaction deficits observed in young children with autism: A randomized clinical crossover trial. Mol. Psychiatry 2016; 21: 1225–1231.
Abstract
Interventions for autism are limited. The synthetic hormone oxytocin may provide a potential treatment to improve core social and behavioral difficulties in autism, but its efficacy has yet to be evaluated in young children who potentially may benefit to a greater extent. We investigated the efficacy, tolerability and safety of oxytocin treatment in young children with autism using a double-blind, randomized, placebo-controlled, crossover, clinical trial. Thirty-one children with autism received 12 International Units (IU) of oxytocin and placebo nasal spray morning and night (24 IU per day) for 5 weeks, with a 4-week washout period between each treatment. Compared with placebo, oxytocin led to significant improvements on the primary outcome of caregiver-rated social responsiveness. Overall, nasal spray was well tolerated, and the most common reported adverse events were thirst, urination and constipation. This study is the first clinical trial to support the potential of oxytocin as an early intervention for young children with autism to help improve social interaction deficits.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4995545/pdf/mp2015162a.pdf
Greene RK, et al. The effects of intranasal oxytocin on reward circuitry responses in children with autism spectrum disorder. J Neurodev Disord 2018; 10: 12.
Abstract
Background: Intranasal oxytocin (OT) has been shown to improve social communication functioning of individuals with autism spectrum disorder (ASD) and, thus, has received considerable interest as a potential ASD therapeutic agent. Although preclinical research indicates that OT modulates the functional output of the mesocorticolimbic dopamine system that processes rewards, no clinical brain imaging study to date has examined the effects of OT on this system using a reward processing paradigm. To address this, we used an incentive delay task to examine the effects of a single dose of intranasal OT, versus placebo (PLC), on neural responses to social and nonsocial rewards in children with ASD.
Methods: In this placebo-controlled double-blind study, 28 children and adolescents with ASD (age: M = 13.43 years, SD = 2.36) completed two fMRI scans, one after intranasal OT administration and one after PLC administration. During both scanning sessions, participants completed social and nonsocial incentive delay tasks. Task-based neural activation and connectivity were examined to assess the impact of OT relative to PLC on mesocorticolimbic brain responses to social and nonsocial reward anticipation and outcomes.
Results: Central analyses compared the OT and PLC conditions. During nonsocial reward anticipation, there was greater activation in the right nucleus accumbens (NAcc), left anterior cingulate cortex (ACC), bilateral orbital frontal cortex (OFC), left superior frontal cortex, and right frontal pole (FP) during the OT condition relative to PLC. Alternatively, during social reward anticipation and outcomes, there were no significant increases in brain activation during the OT condition relative to PLC. A Treatment Group × Reward Condition interaction revealed relatively greater activation in the right NAcc, right caudate nucleus, left ACC, and right OFC during nonsocial relative to social reward anticipation during the OT condition relative to PLC. Additionally, these analyses revealed greater activation during nonsocial reward outcomes during the OT condition relative to PLC in the right OFC and left FP. Finally, functional connectivity analyses generally revealed changes in frontostriatal connections during the OT condition relative to PLC in response to nonsocial, but not social, rewards.
Conclusions: The effects of intranasal OT administration on mesocorticolimbic brain systems that process rewards in ASD were observable primarily during the processing of nonsocial incentive salience stimuli. These findings have implications for understanding the effects of OT on neural systems that process rewards, as well as for experimental trials of novel ASD treatments developed to ameliorate social communication impairments in ASD.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5870086/pdf/11689_2018_Article_9228.pdf
Watanabe T, Abe O, Kuwabara H, et al. Mitigation of sociocommunicational deficits of autism through oxytocin-induced recovery of medial prefrontal activity: a randomized trial. JAMA Psychiatry 2014; 71: 166–75.
Abstract
Importance: Sociocommunicational deficits make it difficult for individuals with autism spectrum disorders (ASD) to understand communication content with conflicting verbal and nonverbal information. Despite growing prospects for oxytocin as a therapeutic agent for ASD, no direct neurobiological evidence exists for oxytocin’s beneficial effects on this core symptom of ASD. This is slowing clinical application of the neuropeptide.
Objective: To directly examine whether oxytocin has beneficial effects on the sociocommunicational deficits of ASD using both behavioral and neural measures.
Design, setting, and participants: At the University of Tokyo Hospital, we conducted a randomized, double-blind, placebo-controlled, within-subject-crossover, single-site experimental trial in which intranasal oxytocin and placebo were administered. A total of 40 highly functioning men with ASD participated and were randomized in the trial.
Interventions: Single-dose intranasal administration of oxytocin (24 IU) and placebo.
Main outcomes and measures: Using functional magnetic resonance imaging, we examined effects of oxytocin on behavioral neural responses of the participants to a social psychological task. In our previous case-control study using the same psychological task, when making decisions about social information with conflicting verbal and nonverbal contents, participants with ASD made judgments based on nonverbal contents less frequently with longer time and could not induce enough activation in the medial prefrontal cortex. Therefore, our main outcomes and measures were the frequency of the nonverbal information-based judgments (NVJs), the response time for NVJs, and brain activity of the medial prefrontal cortex during NVJs.
Results: Intranasal oxytocin enabled the participants to make NVJs more frequently (P = .03) with shorter response time (P = .02). During the mitigated behavior, oxytocin increased the originally diminished brain activity in the medial prefrontal cortex (P < .001). Moreover, oxytocin enhanced functional coordination in the area (P < .001), and the magnitude of these neural effects was predictive of the behavioral effects (P ≤ .01).
Conclusions and relevance: These findings provide the first neurobiological evidence for oxytocin’s beneficial effects on sociocommunicational deficits of ASD and give us the initial account for neurobiological mechanisms underlying any beneficial effects of the neuropeptide.
https://jamanetwork.com/journals/jamapsychiatry/fullarticle/1790357
Anagnostou E, et al. Intranasal oxytocin versus placebo in the treatment of adults with autism spectrum disorders: a randomized controlled trial. Mol Autism 2012;3:16.
Abstract
Background: There are no effective medications for the treatment of social cognition/function deficits in autism spectrum disorder (ASD), and adult intervention literature in this area is sparse. Emerging data from animal models and genetic association studies as well as early, single-dose intervention studies suggest that the oxytocin system may be a potential therapeutic target for social cognition/function deficits in ASD. The primary aim of this study was to examine the safety/therapeutic effects of intranasal oxytocin versus placebo in adults with ASD, with respect to the two core symptom domains of social cognition/functioning and repetitive behaviors.
Methods: This was a pilot, randomized, double-blind, placebo-controlled, parallel design trial of intranasal oxytocin versus placebo in 19 adults with ASD (16 males; 33.20 ± 13.29 years). Subjects were randomized to 24 IU intranasal oxytocin or placebo in the morning and afternoon for 6 weeks. Measures of social function/cognition (the Diagnostic Analysis of Nonverbal Accuracy) and repetitive behaviors (Repetitive Behavior Scale Revised) were administered. Secondary measures included the Social Responsiveness Scale, Reading-the-Mind-in-the-Eyes Test and the Yale Brown Obsessive Compulsive Scale – compulsion subscale and quality of life (World Health Organization Quality of Life Questionnaire – emotional/social subscales). Full-information maximum-likelihood parameter estimates were obtained and tested using mixed-effects regression analyses.
Results: Although no significant changes were detected in the primary outcome measures after correcting for baseline differences, results suggested improvements after 6 weeks in measures of social cognition (Reading-the Mind-in-the-Eyes Test, p = 0.002, d = 1.2), and quality of life (World Health Organization Quality of Life Questionnaire – emotion, p = 0.031, d = 0.84), both secondary measures. Oxytocin was well tolerated and no serious adverse effects were reported.
Conclusions: This pilot study suggests that there is therapeutic potential to daily administration of intranasal oxytocin in adults with ASD and that larger and longer studies are warranted.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3539865/pdf/2040-2392-3-16.pdf
Wirth MM, et al. Effects of intranasal oxytocin on steroid hormones in men and women. Neurophychobiology. 20125;71:202-11.
Abstract
Background: Recent interest in the social and cognitive effects of intranasal oxytocin prompts a need for understanding its physiological effects in humans. Few studies have examined the effects of intranasal oxytocin on steroid hormones. Filling this gap is especially important given the evidence that steroid hormones participate in some of the same behavioral functions as oxytocin, e.g. in stress, processing of emotional stimuli, aggression, trust, empathy, and parental care.
Methods: In randomized, double-blind experiments, we administered oxytocin (24 IU) or saline placebo to 97 healthy participants. Saliva samples were collected before and at several time points after the oxytocin/placebo administration to assess the levels of cortisol, progesterone, and testosterone.
Results: Oxytocin had no effects on testosterone, progesterone, or cortisol in women or men.
Conclusion: Acute intranasal oxytocin does not affect the levels of cortisol, testosterone or progesterone in humans, at least in the absence of a stressful context. These data suggest that acute oxytocin does not have a direct impact on the human hypothalamic-pituitary-adrenal or hypothalamic-pituitarygonadal axes under nonstressful circumstances. This knowledge helps rule out potential mechanisms for some of the effects of oxytocin in humans and adds to the generally limited body of knowledge on the basic physiological or psychological effects of intranasal oxytocin in human beings.
https://psychology.nd.edu/assets/227644/wirth_gaffey_martinez_2015_oxytocin_steroid_hormones.pdf
Gaustella AJ, et al. A randomized controlled trial of intranasal oxytocin as an adjunct to exposure therapy for social anxiety disorder. Psychoneuroendocrinol. 2009;34(6):917-23.
Abstract
In humans, oxytocin nasal administration reduces social-threat perception and improves processes involved in communication and the encoding of positive social cues. The aim of this study was to determine whether oxytocin given as an adjunct to exposure therapy improves treatment for social anxiety disorder (SAD) as indicated by a comprehensive set of symptom outcome measures. In a randomized, double-blind, placebo-controlled trial, we administered 24 IU of oxytocin or a placebo in combination with exposure therapy to twentyfive participants who met primary diagnosis for SAD. Participants administered with oxytocin showed improved positive evaluations of appearance and speech performance as exposure treatment sessions progressed. These effects did not generalize to improve overall treatment outcome from exposure therapy. Participants who received oxytocin or placebo reported similar levels of symptom reduction following treatment across symptom severity, dysfunctional cognition, and life-impairment measures. This study shows that the administration of oxytocin improves mental representations of self, following exposure therapy. These effects may be either short term or situation specific. Future research is now needed to determine whether oxytocin can enhance treatment outcomes for SAD when used with greater frequency, with a wider variety of social learning experiences, and in conjunction with interventions that more specifically target change in broader dysfunctional cognitions.
https://www.psychiatry.wisc.edu/courses/Nitschke/seminar/Guastella%20AJ,%20PNE%2034,%202009.pdf
Sikich L, et al. Intranasal Oxytocin in Children and Adolescents with Autism Spectrum Disorder. N Eng J Med. 2021;385(16):1462-1473.
Abstract
Background: Experimental studies and small clinical trials have suggested that treatment with intranasal oxytocin may reduce social impairment in persons with autism spectrum disorder. Oxytocin has been administered in clinical practice to many children with autism spectrum disorder.
Methods: We conducted a 24-week, placebo-controlled phase 2 trial of intranasal oxytocin therapy in children and adolescents 3 to 17 years of age with autism spectrum disorder. Participants were randomly assigned in a 1:1 ratio, with stratification according to age and verbal fluency, to receive oxytocin or placebo, administered intranasally, with a total target dose of 48 international units daily. The primary outcome was the least-squares mean change from baseline on the Aberrant Behavior Checklist modified Social Withdrawal subscale (ABC-mSW), which includes 13 items (scores range from 0 to 39, with higher scores indicating less social interaction). Secondary outcomes included two additional measures of social function and an abbreviated measure of IQ.
Results: Of the 355 children and adolescents who underwent screening, 290 were enrolled. A total of 146 participants were assigned to the oxytocin group and 144 to the placebo group; 139 and 138 participants, respectively, completed both the baseline and at least one postbaseline ABC-mSW assessments and were included in the modified intention-to-treat analyses. The least-squares mean change from baseline in the ABC-mSW score (primary outcome) was -3.7 in the oxytocin group and -3.5 in the placebo group (least-squares mean difference, -0.2; 95% confidence interval, -1.5 to 1.0; P = 0.61). Secondary outcomes generally did not differ between the trial groups. The incidence and severity of adverse events were similar in the two groups.
Conclusions: This placebo-controlled trial of intranasal oxytocin therapy in children and adolescents with autism spectrum disorder showed no significant between-group differences in the least-squares mean change from baseline on measures of social or cognitive functioning over a period of 24 weeks.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9701092/pdf/nihms-1849726.pdf
Behinia B, et al. Differential effects of intranasal oxytocin on sexual experiences and partner interactions in couples. Horm Behav. 2014;65(3):308-18.
Abstract
Knowledge about the effects of the neuropeptide oxytocin (OXT) on human sexual behaviors and partner interactions remains limited. Based on our previous studies, we hypothesize that OXT should be able to positively influence parameters of sexual function and couple interactions. Employing a naturalistic setting involving 29 healthy heterosexual couples (n=58 participants), we analyzed the acute effects of intranasally administered OXT (24IU) on sexual drive, arousal, orgasm and refractory aspects of sexual behavior together with partner interactions. Data were assessed by psychometric instruments (Acute Sexual Experiences Scale, Arizona Sexual Experience Scale) as well as biomarkers, such as cortisol, α-amylase and heart rate. Intranasal OXT administration did not alter “classical” parameters of sexual function, such as sexual drive, arousal or penile erection and lubrication. However, analysis of variance and a hierarchical linear model (HLM) revealed specific effects related to the orgasmic/post-orgasmic interval as well as parameters of partner interactions. According to HLM analysis, OXT increased the intensity of orgasm, contentment after sexual intercourse and the effect of study participation. According to ANOVA analysis, these effects were more pronounced in men. Men additionally indicated higher levels of sexual satiety after sexual intercourse with OXT administration. Women felt more relaxed and subgroups indicated better abilities to share sexual desires or to empathize with their partners. The effect sizes were small to moderate. Biomarkers indicated moderate psychophysiological activation but were not affected by OXT, gender or method of contraception. Using a naturalistic setting, intranasal OXT administration in couples exerted differential effects on parameters of sexual function and partner interactions. These results warrant further investigations, including subjects with sexual and relationship problems.
No Full Text Available
Rash JA, et al. Evaluating the efficacy of intranasal oxytocin on pain and function among individuals who experience chronic pain: a protocol for a multisite, placebo-controlled, blinded, sequential, within subjects crossover trial. BMJ Open. 2021;11:e055039.
Abstract
Introduction: Current treatments for chronic pain (eg, opioids) can have adverse side effects and rarely result in resolution of pain. As such, there is a need for adjuvant analgesics that are non-addictive, have few adverse side effects and are effective for pain management across several chronic pain conditions. Oxytocin is a naturally occurring hormone that has gained attention for its potential analgesic properties. The objective of this trial is to evaluate the efficacy of intranasal oxytocin on pain and function among adults with chronic pain.
Methods and analysis: This is a placebo-controlled, triple-blind, sequential, within-subject crossover trial. Adults with chronic neuropathic, pelvic and musculoskeletal pain will be recruited from three Canadian provinces (British Columbia, Alberta and Newfoundland and Labrador, respectively). Enrolled patients will provide one saliva sample pretreatment to evaluate basal oxytocin levels and polymorphisms of the oxytocin receptor gene before being randomised to one of two trial arms. Patients will self-administer three different oxytocin nasal sprays twice daily for a period of 2 weeks (ie, 24 IU, 48 IU and placebo). Patients will complete daily diaries, including standardised measures on day 1, day 7 and day 14. Primary outcomes include pain and pain-related interference. Secondary outcomes include emotional function, sleep disturbance and global impression of change. Intention-to-treat analyses will be performed to evaluate whether improvement in pain and physical function will be observed posttreatment.
Ethics and dissemination: Trial protocols were approved by the Newfoundland and Labrador Health Research Ethics Board (HREB #20227), University of British Columbia Clinical Research Ethics Board (CREB #H20-00729), University of Calgary Conjoint Health Research Ethics Board (REB20 #0359) and Health Canada (Control # 252780). Results will be disseminated through publication in peer-reviewed journals and presentations at scientific conferences.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8461687/pdf/bmjopen-2021-055039.pdf
Lawson EA, et al. Oxytocin reduces caloric intake in men. Obesity (Silver Spring) 2015;23:950–6.
Abstract
Objective: Preclinical studies indicate that oxytocin is anorexigenic and has beneficial metabolic effects. Oxytocin effects on nutrition and metabolism in humans are not well defined. We hypothesized that oxytocin would reduce caloric intake and appetite, and alter levels of appetite-regulating hormones. We also explored metabolic effects of oxytocin.
Methods: We performed a randomized, placebo-controlled crossover study of single-dose intranasal oxytocin (24 IU) in 25 fasting healthy men. After oxytocin/placebo, subjects selected breakfast from a menu, and were given double portions. Caloric content of food consumed was measured. Visual analogue scales were used to assess appetite and blood was drawn for appetite-regulating hormones, insulin, and glucose before and after oxytocin/placebo. Indirect calorimetry assessed resting energy expenditure (REE) and substrate utilization.
Results: Oxytocin reduced caloric intake with a preferential effect on fat intake and increased levels of the anorexigenic hormone cholecystokinin without affecting appetite or other appetite-regulating hormones. There was no effect of oxytocin on REE. Oxytocin resulted in a shift from carbohydrate to fat utilization and improved insulin sensitivity.
Conclusions: Intranasal oxytocin reduces caloric intake and has beneficial metabolic effects in men without concerning side effects. The efficacy and safety of sustained oxytocin administration in the treatment of obesity warrants investigation.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4414748/pdf/nihms-662890.pdf
Cacciotti-Saija C, et al. A Double-Blind Randomized Controlled Trial of Oxytocin Nasal Spray and Social Cognition Training for Young People With Early Psychosis. Scizophren Bull. 2015;41(2):483-93.
Abstract
Social-cognitive deficits contribute to poor functional outcomes in early psychosis; however, no effective pharmacological treatments exist for these problems. This study was the first to investigate the efficacy of an extended treatment of oxytocin nasal spray combined with social cognition training (SCT) to improve social cognition, clinical symptoms, and social functioning in early psychosis. In a double-blind, randomized, placebo-controlled, between-subjects trial, 52 individuals (aged 16-35 years) diagnosed with an early psychosis schizophrenia-spectrum illness were recruited. Participants received oxytocin (24 International Units) or placebo nasal spray twice-daily for 6 weeks, combined with group SCT (2 × 1 hour weekly sessions for 6 weeks). An additional dose of oxytocin was administered before each weekly session. Assessments were conducted at baseline, post-treatment, and at 3-month follow-up. Primary outcomes included the Reading the Mind in the Eyes Test, the Scale for the Assessment of Positive and Negative Symptoms, and the Social Functioning Scale. Secondary outcomes included self-report and behavioral assessments of social cognition, symptom severity, and social functioning. Results showed that on all primary and secondary outcomes, there was no benefit of oxytocin nasal spray treatment in comparison to placebo. Exploratory post hoc analysis suggested that increased use of nasal spray was, however, associated with reductions in negative symptoms in the oxytocin condition only. This study represents the first evaluation of oxytocin treatment for early psychosis. Although results suggest no benefit of oxytocin treatment, results also highlight an urgent need to consider nasal spray delivery and dose-related variables for future clinical trials.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4332939/pdf/sbu094.pdf
Muin DA, et al. Effect of long-term intranasal oxytocin on sexual dysfunction in premenopausal and postmenopausal women: a randomized trial. Fertil Steril. 2015;104(3):715-23.
Abstract
Objective: To assess the effect of on-demand intranasal oxytocin administration on female sexual function and activity.
Design: Randomized, prospective, double-blind, placebo-controlled, crossover trial with duration of 22 weeks.
Setting: Academic medical center.
Patient(s): Thirty pre-and postmenopausal women with sexual dysfunction.
Intervention(s): Over 8 weeks, intranasal oxytocin (32 IU) or placebo self-administered by women within 50 minutes before sexual intercourse; after a washout period of 2 weeks, crossover with patients switched to the alternate group for another 8 weeks.
Main Outcome Measure(s): Primary outcome parameter: Female Sexual Function Index (FSFI); secondary outcome parameters: Female Sexual Distress Scale (FSDS), Sexual Quality of Life–Female (SQOL-F), Sexual Interest and Desire Inventory–Female (SIDI-F), and Hamilton depression scale (HDS).
Result(s): After oxytocin and placebo, the FSFI score increased by 26% and 31%, SQOL-F score by 144% and 125%, and SIDI-F score by 29% and 23%, respectively (repeated measures analysis of variance between groups). After oxytocin and placebo, the FSDS score decreased by 36% and 45%, respectively (repeated measures analysis of variance between groups). There was no statistically significant treatment, sequence (placebo first/second), or interaction effect.
Conclusion(s): Long-term intranasal oxytocin and placebo administration both improved sexual function and symptoms of depression in women over time with no treatment, sequence (placebo first/second), or interaction effect.
https://www.fertstert.org/action/showPdf?pii=S0015-0282%2815%2900432-X
Level 4
Averbeck BB, et al. Emotion recognition and oxytocin in patients with schizophrenia. Psychol Med. 2012;42(2):259-66.
Abstract
Background: Studies have suggested that patients with schizophrenia are impaired at recognizing emotions. Recently, it has been shown that the neuropeptide oxytocin can have beneficial effects on social behaviors.
Method: To examine emotion recognition deficits in patients and see whether oxytocin could improve these deficits, we carried out two experiments. In the first experiment we recruited 30 patients with schizophrenia and 29 age- and IQ-matched control subjects, and gave them an emotion recognition task. Following this, we carried out a second experiment in which we recruited 21 patients with schizophrenia for a double-blind, placebo-controlled cross-over study of the effects of oxytocin on the same emotion recognition task.
Results: In the first experiment we found that patients with schizophrenia had a deficit relative to controls in recognizing emotions. In the second experiment we found that administration of oxytocin improved the ability of patients to recognize emotions. The improvement was consistent and occurred for most emotions, and was present whether patients were identifying morphed or non-morphed faces.
Conclusions: These data add to a growing literature showing beneficial effects of oxytocin on social-behavioral tasks, as well as clinical symptoms.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3250086/pdf/S0033291711001413a.pdf
Kou J, et al. In the nose or on the tongue? Contrasting motivational effects of oral and intranasal oxytocin on arousal and reward during social processing. Translational Psychiatry. 2021;11:94.
Abstract
Intranasal oxytocin exerts wide-ranging effects on socioemotional behavior and is proposed as a potential therapeutic intervention in psychiatric disorders. However, following intranasal administration, oxytocin could penetrate directly into the brain or influence its activity via increased peripheral concentrations crossing the blood–brain barrier or influencing vagal projections. In the current randomized, placebo-controlled, pharmaco-imaging clinical trial we investigated effects of 24IU oral (lingual) oxytocin spray, restricting it to peripherally mediated blood-borne and vagal effects, on responses to face emotions in 80 male subjects and compared them with 138 subjects treated intranasally with 24IU. Oral, but not intranasal oxytocin administration increased both arousal ratings for faces and associated brain reward responses, the latter being partially mediated by blood concentration changes. Furthermore, while oral oxytocin increased amygdala and arousal responses to face emotions, after intranasal administration they were decreased. Thus, oxytocin can produce markedly contrasting motivational effects in relation to socioemotional cues when it influences brain function via different routes. These findings have important implications for future therapeutic use since administering oxytocin orally may be both easier and have potentially stronger beneficial effects by enhancing responses to emotional cues and increasing their associated reward.
https://www.nature.com/articles/s41398-021-01241-w#Sec11
Scantamburlo G, et al. Plasma oxytocin levels and anxiety in patients with major depression. Psychoneuroendocrinology 2007;32(4):407–410.
Abstract
Cerebrospinal fluid and plasmatic levels of oxytocin (OT) have been found to change in mood disorders. In post-mortem studies, the numbers of OT-expressing neurons in the paraventricular nucleus have been reported to be increased. Moreover, OT is considered as an endogenous antistress hormone. It has also revealed antidepressive effects. OT may contribute to the dysregulation of the HPA system in major depression. The aim of the study was to assess a possible relationship between anxiety and plasma oxytocin (OT) levels in depressive patients. Severity of depression was estimated with the Hamilton Depression Rating Scale and anxiety by using the Spielberger State-Anxiety Inventory. Results showed a significant negative correlation between oxytocin and the scored symptoms depression (r=-0.58, p=0.003) and anxiety (r=-0.61, p=0.005).
https://orbi.uliege.be/bitstream/2268/6401/1/PlasmaOT.pdf
Striepens N, et al. Elevated cerebrospinal fluid and blood concentrations of oxytocin following its intranasal administration in humans. Sci. Rep. 2013; 3: 3440.
Abstract
There has been an unprecedented interest in the modulatory effects of intranasal oxytocin on human social cognition and behaviour, however as yet no study has actually demonstrated that this modality of administration increases concentrations of the peptide in the brain as well as blood in humans. Here using combined blood and cerebrospinal fluid (CSF) sampling in subjects receiving either 24 IU of oxytocin (n 5 11) or placebo (n 5 4) we have shown that oxytocin levels significantly increased in both plasma and CSF. However, whereas oxytocin plasma concentrations peaked at 15 min after intranasal administration and decreased after 75 min, CSF concentrations took up to 75 min to reach a significant level. Moreover, there was no correlation (r 5 ,0.10) between oxytocin plasma and CSF concentrations. Together, these data provide crucial insights into the plasma and CSF kinetics of intranasally administered oxytocin.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3853684/pdf/srep03440.pdf
Kosfeld M, et al. Oxytocin increases trust in humans. Nature. 2005;435(7042)673-6.
Abstract
Trust pervades human societies. Trust is indispensable in friendship, love, families and organizations, and plays a key role in economic exchange and politics. In the absence of trust among trading partners, market transactions break down. In the absence of trust in a country’s institutions and leaders, political legitimacy breaks down. Much recent evidence indicates that trust contributes to economic, political and social success. Little is known, however, about the biological basis of trust among humans. Here we show that intranasal administration of oxytocin, a neuropeptide that plays a key role in social attachment and affiliation in non-human mammals, causes a substantial increase in trust among humans, thereby greatly increasing the benefits from social interactions. We also show that the effect of oxytocin on trust is not due to a general increase in the readiness to bear risks. On the contrary, oxytocin specifically affects an individual’s willingness to accept social risks arising through interpersonal interactions. These results concur with animal research suggesting an essential role for oxytocin as a biological basis of prosocial approach behaviour.
https://www.zora.uzh.ch/id/eprint/2250/9/2005_KosfeldM_Nature_Oxytocin-incr.pdf
Level 6
Ohlsson B, et al. Oxytocin is expressed throughout the human gastrointestinal tract. Regul Pept. 2006;135:7–11.
Abstract
Background/aim: Several studies have described that oxytocin exerts stimulatory or inhibitory effects on gut functions. Recently, mRNA for oxytocin and its receptor was found throughout the entire human gastrointestinal (GI) tract. The aim of this study was to examine the cellular localization and distribution of the corresponding proteins.
Material and methods: Full-thickness biopsies from 24 patients, covering the entire GI tract, were collected during operations at the Department of Surgery in Malmö and Lund. The biopsies were taken from non_affected margins. The biopsies were fixed by immersion, rinsed in buffered sucrose, and kept frozen at 70 degrees C. Indirect immunofluorescence with primary antibodies to oxytocin and its receptor was used.
Results: Oxytocin was expressed in nerve cell bodies and nerve fibres in the myenteric and submucous ganglia all along the GI tract. Immunoreactive nerve cell bodies in myenteric ganglia predominated in the proximal (antrum and duodenum) and distal gut, while those in the submucous ganglia were more numerous in the ileum and colon. The oxytocin receptor was not detectable by two different antibodies in any tissue in the GI tract.
Conclusion: Oxytocin is expressed in the myenteric and submucous ganglia and nerve fibres along the entire human GI tract. The role for oxytocin in the physiology and pathophysiology of the bowel remains to be settled.
https://lucris.lub.lu.se/ws/portalfiles/portal/2983922/625470.pdf
Level 7
Lukas M, et al. The neuropeptide oxytocin facilitates pro-social behavior and prevents social avoidance in rats and mice. Neuropsychopharmacology. 2011;36(11):2159-68.
Abstract
Social avoidance and social phobia are core symptoms of various psychopathologies but their underlying etiology remains poorly understood. Therefore, this study aims to reveal pro-social effects of the neuropeptide oxytocin (OT), under both basal and stressinduced social avoidance conditions in rodents using a social preference paradigm. We initially show that intracerebroventricular (i.c.v.) application of an OT receptor antagonist (OTR-A) in naı¨ve male rats (0.75 mg/5 ml), or mice (20 mg/2 ml), reduced social exploration of a novel con-specific indicative of attenuated social preference. Previous exposure of male rats to a single social defeat resulted in loss of their social preference and social avoidance, which could be restored by i.c.v. infusion of synthetic OT (0.1 mg/5 ml) 20 min before the social preference test. Although the amygdala has been implicated in both social and OT-mediated actions, bilateral OTR-A (0.1 mg/1 ml) or OT (0.01 mg/1 ml) administration into various subnuclei of the amygdala did not affect basal or stress-induced social preference behavior, respectively. Finally, we demonstrate the social specificity of these OT-mediated effects by showing that neither an arginine vasopressin V1a receptor antagonist (0.75 mg/5 ml, i.c.v.) nor the anxiogenic drug pentylenetetrazol (15 mg/kg, i.p.) altered social preference, with OTR-A not affecting non-social anxiety on the elevated plus-maze. Overall, the data indicate that the basal activity of the endogenous brain OT system is sufficient to promote natural occurring social preference in rodents while synthetic OT shows potential to reverse stress-induced social avoidance and might thus be of use for treating social phobia and social dysfunction in humans.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3176581/pdf/npp201195a.pdf
Freeman SM, et al. Plasma and CSF oxytocin levels after intranasal and intravenous oxytocin in awake macaques. Psychoneuroendocrinol. 2016;66:185-94.
Abstract
Oxytocin (OT) is a neuropeptide that mediates a variety of complex social behaviors in animals and humans. Intranasal OT has been used as an experimental therapeutic for human conditions characterized by deficits in social functioning, especially autism spectrum disorder and schizophrenia. However, it is currently under intense debate whether intranasal delivery of OT reaches the central nervous system. In this study, four female rhesus macaques were implanted with chronic intrathecal catheters and used to investigate the pharmacokinetic profile of OT in the central nervous system and the peripheral vasculature following intravenous (IV) and intranasal (IN) administration of OT. In a randomized, crossover design, OT was given to four awake monkeys at three different doses based on body weight (0.1 IU/kg; 1 IU/kg; 5 IU/kg). A time course of concurrent cerebrospinal fluid (CSF) and plasma samples were taken following administration. We found a dose-dependent effect of IV OT treatment on plasma OT levels, which peaked at 5 min post-dose and gradually returned to baseline by 120 min. In contrast, a change in CSF OT was only observed at the highest IV dose (5 IU/kg) at 15 min post-dose and gradually returned to baseline by 120 min. After IN administration, there was no significant change in plasma OT at any of the three doses. However, at the highest dose level, we found a significant increase in CSF OT at 15–30 min post- dose. The results of this study in light of recent, similar publications highlight the importance of methodological consistency across studies. This study also establishes a non-human primate model that can provide a stable platform for carrying out serial sampling from the central nervous system and peripheral vasculature concurrently.
Li C, et al. Molecular mechanisms of antidiuretic effect of oxytocin. J Am Soc Nephrol. 2008;19(2):225-232.
Abstract
Oxytocin is known to have an antidiuretic effect, but the mechanisms underlying this effect are not completely understood. We infused oxytocin by osmotic minipump into vasopressin-deficient Brattleboro rats for five days and observed marked antidiuresis, increased urine osmolality, and increased solute-free water reabsorption. Administration of oxytocin also significantly increased the protein levels of aquaporin-2 (AQP2), phosphorylated AQP2 (p-AQP2), and AQP3 in the inner medulla and in the outer medulla plus cortex. Immunohistochemistry demonstrated increased AQP2 and p-AQP2 expression and trafficking to the apical plasma membrane of principal cells in the collecting duct, and increased AQP3 expression in the basolateral membrane. These oxytocin-induced effects were blocked by treatment with the vasopressin V2 receptor antagonist SR121463B, but not by treatment with the oxytocin receptor antagonist GW796679X. We conclude that vasopressin V2 receptors mediate the antidiuretic effects of oxytocin, including increased expression and apical trafficking of AQP2, p-AQP2, and increased AQP3 protein expression.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2396735/?report=reader
Melis MR, Argiolas A. Central control of penile erection: a re-visitation of the role of oxytocin and its interaction with dopamine and glutamic acid in male rats. Neurosci Biobehav Rev. 2011;35:939–55.
Abstract
Oxytocin is a potent inducer of penile erection when injected into the central nervous system. In male rats, the most sensitive brain area for the pro-erectile effect of oxytocin is the paraventricular nucleus of the hypothalamus. This nucleus and surrounding regions contain the cell bodies of all oxytocinergic neurons projecting to extra-hypothalamic brain areas and the spinal cord. This review shows that oxytocin induces penile erection also when injected in some of these areas (e.g., ventral tegmental area, ventral subiculum of the hippocampus, posteromedial cortical nucleus of the amygdala and thoraco-lumbar spinal cord). Microinjection studies combined with intra-cerebral microdialysis and double immuno-fluorescence studies suggest that oxytocin in these areas activates directly or indirectly (mainly through glutamic acid) mesolimbic dopaminergic neurons. Dopamine released in the nucleus accumbens in turn activates neural pathways leading to the activation of incerto-hypothalamic dopaminergic neurons in the paraventricular nucleus. This activates not only oxytocinergic neurons projecting to the spinal cord and mediating penile erection, but also those projecting to the above extra-hypothalamic areas, modulating directly or indirectly (through glutamic acid) the activity of mesolimbic dopaminergic neurons controlling motivation and reward. Together these neural pathways may constitute a complex hypothetical circuit, which plays a role not only in the consummatory phase of sexual activity (erectile function and copulation), but also in the motivational and rewarding aspects of the anticipatory phase of sexual behaviour.
Full Text Not Available
Neumann ID, et al. Increased brain and plasma oxytocin after nasal and peripheral administration in rats and mice. Psychoneuroendocrinol. 2013;38(10):1985-93.
Abstract
The possibility to improve socio-emotional behaviors in humans by intranasal administration of synthetic oxytocin (OXT) attracts increasing attention, but its uptake into the brain has never been demonstrated so far. Here we used simultaneous microdialysis in both the dorsal hippocampus and amygdala of rats and mice in combination with concomitant blood sampling from the jugular vein to study the dynamics of the neuropeptide in brain extracellular fluid and plasma after its nasal administration. OXT was found to be increased in microdialysates from both the hippocampus and amygdala with peak levels occurring 30-60min after nasal administration. Despite a similar temporal profile of OXT concentrations in plasma, peripheral OXT is unlikely to contribute to dialysate OXT as calculated from in vitro recovery data, indicating a central route of transport. Moreover, intraperitoneal administration of synthetic OXT in identical amounts caused rapid peak levels in brain dialysates and plasma during the first 30min after treatment and a subsequent return toward baseline. While the precise route(s) of central transport remain to be elucidated, our data provide the first evidence that nasally applied OXT indeed reaches behaviorally relevant brain areas, and this uptake is paralleled by changes in plasma OXT.
No Full Text Available
Phie J, et al. Prolonged Subcutaneous Administration of Oxytocin Accelerates Angiotensin II-Induced Hypertension and Renal Damage in Male Rats. PLoS One. 2015;10(9):e0138048.
Abstract
Oxytocin and its receptor are synthesised in the heart and blood vessels but effects of chronic activation of this peripheral oxytocinergic system on cardiovascular function are not known. In acute studies, systemic administration of low dose oxytocin exerted a protective, preconditioning effect in experimental models of myocardial ischemia and infarction. In this study, we investigated the effects of chronic administration of low dose oxytocin following angiotensin II-induced hypertension, cardiac hypertrophy and renal damage. Angiotensin II (40 μg/Kg/h) only, oxytocin only (20 or 100 ng/Kg/h), or angiotensin II combined with oxytocin (20 or 100 ng/Kg/h) were infused subcutaneously in adult male Sprague-Dawley rats for 28 days. At day 7, oxytocin or angiotensin-II only did not change hemodynamic parameters, but animals that received a combination of oxytocin and angiotensin-II had significantly elevated systolic, diastolic and mean arterial pressure compared to controls (P < 0.01). Hemodynamic changes were accompanied by significant left ventricular cardiac hypertrophy and renal damage at day 28 in animals treated with angiotensin II (P < 0.05) or both oxytocin and angiotensin II, compared to controls (P < 0.01). Prolonged oxytocin administration did not affect plasma concentrations of renin and atrial natriuretic peptide, but was associated with the activation of calcium-dependent protein phosphatase calcineurin, a canonical signalling mechanism in pressure overload-induced cardiovascular disease. These data demonstrate that oxytocin accelerated angiotensin-II induced hypertension and end-organ renal damage, suggesting caution should be exercised in the chronic use of oxytocin in individuals with hypertension.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4579129/pdf/pone.0138048.pdf
Level 8
Yao S, Kendrick KM. Effects of Intranasal Administration of Oxytocin and Vasopressin on Social Cognition and Potential Routes and Mechanisms of Action. Pharmaceutics. 2022;14(2):323.
Abstract
Acute and chronic administration of intranasal oxytocin and vasopressin have been extensively utilized in both animal models and human preclinical and clinical studies over the last few decades to modulate various aspects of social cognition and their underlying neural mechanisms, although effects are not always consistent. The use of an intranasal route of administration is largely driven by evidence that it permits neuropeptides to penetrate directly into the brain by circumventing the blood–brain barrier, which has been considered relatively impermeable to them. However, this interpretation has been the subject of considerable debate. In this review, we will focus on research in both animal models and humans, which investigates the different potential routes via which these intranasally administered neuropeptides may be producing their various effects on social cognition. We will also consider the contribution of different methods of intranasal application and additionally the importance of dose magnitude and frequency for influencing G protein-coupled receptor signaling and subsequent functional outcomes. Overall, we conclude that while some functional effects of intranasal oxytocin and vasopressin in the domain of social cognition may result from direct penetration into the brain following intranasal administration, others may be contributed by the neuropeptides either entering the peripheral circulation and crossing the blood–brain barrier and/or producing vagal stimulation via peripheral receptors. Furthermore, to complicate matters, functional effects via these routes may differ, and both dose magnitude and frequency can produce very different functional outcomes and therefore need to be optimized to produce desired effects.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8874551/pdf/pharmaceutics-14-00323.pdf
Carter CS, et al. Is oxytocin “nature’s medicine”? Pharmacol Rev. 2020;72(4):829-61.
Abstract
Oxytocin is a pleiotropic, peptide hormone with broad implications for general health, adaptation, development, reproduction, and social behavior. Endogenous oxytocin and stimulation of the oxytocin receptor support patterns of growth, resilience, and healing. Oxytocin can function as a stress-coping molecule, an anti-inflammatory, and an antioxidant, with protective effects especially in the face of adversity or trauma. Oxytocin influences the autonomic nervous system and the immune system. These properties of oxytocin may help explain the benefits of positive social experiences and have drawn attention to this molecule as a possible therapeutic in a host of disorders. However, as detailed here, the unique chemical properties of oxytocin, including active disulfide bonds, and its capacity to shift chemical forms and bind to other molecules make this molecule difficult to work with and to measure. The effects of oxytocin also are context-dependent, sexually dimorphic, and altered by experience. In part, this is because many of the actions of oxytocin rely on its capacity to interact with the more ancient peptide molecule, vasopressin, and the vasopressin receptors. In addition, oxytocin receptor(s) are epigenetically tuned by experience, especially in early life. Stimulation of G-protein-coupled receptors triggers subcellular cascades allowing these neuropeptides to have multiple functions. The adaptive properties of oxytocin make this ancient molecule of special importance to human evolution as well as modern medicine and health; these same characteristics also present challenges to the use of oxytocin-like molecules as drugs that are only now being recognized. SIGNIFICANCE STATEMENT: Oxytocin is an ancient molecule with a major role in mammalian behavior and health. Although oxytocin has the capacity to act as a “natural medicine” protecting against stress and illness, the unique characteristics of the oxytocin molecule and its receptors and its relationship to a related hormone, vasopressin, have created challenges for its use as a therapeutic drug.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7495339/pdf/pr.120.019398.pdf
Guastella AJ, et al. Recommendations for the standardisation of oxytocin nasal administration and guidelines for its reporting in human research. Psychoneuroendocrinol. 2013;38(5):612-25.
Abstract
A series of studies have reported on the salubrious effects of oxytocin nasal spray on social cognition and behavior in humans, across physiology (e.g., eye gaze, heart rate variability), social cognition (e.g., attention, memory, and appraisal), and behavior (e.g., trust, generosity). Findings suggest the potential of oxytocin nasal spray as a treatment for various psychopathologies, including autism and schizophrenia. There are, however, increasing reports of variability of response to oxytocin nasal spray between experiments and individuals. In this review, we provide a summary of factors that influence transmucosal nasal drug delivery, deposition, and their impact on bioavailability. These include variations in anatomy and resultant airflow dynamic, vascularisation, status of blood vessels, mode of spray application, gallenic formulation (including presence of uptake enhancers, control release formulation), and amount and method of administration. These key variables are generally poorly described and controlled in scientific reports, in spite of their potential to alter the course of treatment outcome studies. Based on this review, it should be of no surprise that differences emerge across individuals and experiments when nasal drug delivery methods are employed. We present recommendations for researchers to use when developing and administering the spray, and guidelines for reporting on peptide nasal spray studies in humans. We hope that these recommendations assist in establishing a scientific standard that can improve the rigor and subsequent reliability of reported effects of oxytocin nasal spray in humans.
No Full Text Available
Shahrestani S, et al. The Impact of a Single Administration of Intranasal Oxytocin on the Recognition of Basic Emotions in Humans: A Meta-Analysis. Neuropsychopharmacol. 2013;38(10):1929-36.
Abstract
Many studies have highlighted the potential of oxytocin (OT) to enhance facial affect recognition in healthy humans. However, inconsistencies have emerged with regard to the influence of OT on the recognition of specific emotional expressions (happy, angry, fear, surprise, disgust, and sadness). In this study, we conducted a meta-analysis of seven studies comprising 381 research participants (71 females) examining responses to the basic emotion types to assess whether OT enhances the recognition of emotion from human faces and whether this was influenced by the emotion expression and exposure time of the face. Results showed that intranasal OT administration enhances emotion recognition of faces overall, with a Hedges g effect size of 0.29. When analysis was restricted to facial expression types, significant effects of OT on recognition accuracy were specifically found for the recognition of happy and fear faces. We also found that effect sizes increased to moderate when exposure time of the photograph was restricted to early phase recognition (< 300 ms) for happy and angry faces, or later phase recognition for fear faces (> 300 ms). The results of the meta-analysis further suggest that OT has potential as a treatment to improve the recognition of emotion in faces, allowing individuals to improve their insight into the intentions, desires, and mental states of others.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3746698/pdf/npp201386a.pdf
ALeslie M, Silva P, Paloyelis Y, Blevins J, Treasure J. A systematic review and quantitative meta-analysis of Oxytocin’s effects on feeding. J Neuroendocrinol. 2018;30:e12584.
Abstract
Purpose: Oxytocin’s anorexigenic effects have been widely documented and accepted; however, no paper has yet used the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) guidelines to compile previous findings in a single systematic review and quantitative meta-analysis. The current paper aimed to identify published and unpublished studies examining the effects of oxytocin on energy intake in animals and humans, and the factors that moderate this effect.
Methods: Web of Science, Pub Med, and Ovid were searched for published and unpublished studies reporting the effects of oxytocin on energy intake in wild-type animals and in humans, when administered in the absence of other active drugs or surgery.
Results: 2049 articles were identified through the original systematic literature search, from which 54 articles were identified as relevant for inclusion in this review. An additional 3 relevant articles were identified in a later update of the literature search. Overall, a single-dose of oxytocin was found to reduce feeding in animals. Despite several individual studies which found that this effect persists to the end of the third week of chronic administration in rodent models, overall, this anorexigenic effect did not hold in the meta-analyses testing the effects of chronic administration. There was no overall effect of oxytocin on energy intake in humans, although a trend was identified for oxytocin to reduce consumption of solid foods.
Conclusions: Oxytocin reduces energy intake when administered as a single dose. Oxytocin can inhibit feeding over two- to three-week periods in rodent models. These effects typically do not persist beyond the third week of treatment. The anorexigenic effect of oxytocin is moderated by pregnant status, dose, method of administration, and diet composition.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6292740/pdf/nihms-995311.pdf
Zhang H, Wu C, Chen Q, Chen X, Xu Z, Wu J, et al. Treatment of obesity and diabetes using oxytocin or analogs in patients and mouse models. PLoS One. 2013;8:e61477.
Abstract
Obesity is important for the development of type-2 diabetes as a result of obesity-induced insulin resistance accompanied by impaired compensation of insulin secretion from pancreatic beta cells. Here, based on a randomized pilot clinical trial, we report that intranasal oxytocin administration over an 8-week period led to effective reduction of obesity and reversal of related prediabetic changes in patients. Using mouse models, we further systematically evaluated whether oxytocin and its analogs yield therapeutic effects against prediabetic or diabetic disorders regardless of obesity. Our results showed that oxytocin and two analogs including [Ser4, Ile8]-oxytocin or [Asu1,6]-oxytocin worked in mice to reverse insulin resistance and glucose intolerance prior to reduction of obesity. In parallel, using streptozotocin-induced diabetic mouse model, we found that treatment with oxytocin or its analogs reduced the magnitude of glucose intolerance through improving insulin secretion. The anti-diabetic effects of oxytocin and its analogs in these animal models can be produced similarly whether central or peripheral administration was used. In conclusion, oxytocin and its analogs have multi-level effects in improving weight control, insulin sensitivity and insulin secretion, and bear potentials for being developed as therapeutic peptides for obesity and diabetes.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3658979/pdf/pone.0061477.pdf
Ott V, et al. Oxytocin reduces reward-driven food intake in humans. Diabetes. 2013;62:3418–25.
Abstract
Experiments in animals suggest that the neuropeptide oxytocin acts as an anorexigenic signal in the central nervous control of food intake. In humans, however, research has almost exclusively focused on the involvement of oxytocin in the regulation of social behavior. We investigated the effect of intranasal oxytocin on ingestion and metabolic function in healthy men. Food intake in the fasted state was examined 45 min after neuropeptide administration, followed by the assessment of olfaction and reward-driven snack intake in the absence of hunger. Energy expenditure was registered by indirect calorimetry, and blood was repeatedly sampled to determine concentrations of blood glucose and hormones. Oxytocin markedly reduced snack consumption, restraining, in particular, the intake of chocolate cookies by 25%. Oxytocin, moreover, attenuated basal and postprandial levels of adrenocorticotropic hormone and cortisol and curbed the meal-related rise in plasma glucose. Energy expenditure and hunger-driven food intake as well as olfactory function were not affected. Our results indicate that oxytocin, beyond its role in social bonding, regulates nonhomeostatic, reward-related energy intake, hypothalamic-pituitary-adrenal axis activity, and the glucoregulatory response to food intake in humans. These effects can be assumed to converge with the psychosocial function of oxytocin and imply possible applications in the treatment of metabolic disorders.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3781467/pdf/3418.pdf
Tracy LM, Georgiou-Karistianis N, Gibson SJ, et al. Oxytocin and the modulation of pain experience: implications for chronic pain management. Neurosci Biobehav Rev. 2015;55:53–67.
Abstract
In an acute environment pain has potential protective benefits. However when pain becomes chronic this protective effect is lost and the pain becomes an encumbrance. Previously unheralded substances are being investigated in an attempt to alleviate the burden of living with chronic pain. Oxytocin, a neuropeptide hormone, is one prospective pharmacotherapeutic agent gaining popularity. Oxytocin has the potential to modulate the pain experience due to its ubiquitous involvement in central and peripheral psychological and physiological processes, and thus offers promise as a therapeutic agent. In this review, we discuss previous effective applications of oxytocin in pain-free clinical populations and its potential use in the modulation of pain experience. We also address the slowly growing body of literature investigating the administration of oxytocin in clinical and experimentally induced pain in order to investigate the potential mechanisms of its reported analgesic actions. We conclude that oxytocin offers a potential novel avenue for modulating the experience of pain, and that further research into this area is required to map its therapeutic benefit.
Rash JA, et al. Oxytocin and pain: a systematic review and synthesis of findings. Clin J Pain 2014;30:453–62.
Abstract
Objectives: A review of the literature was conducted to assess the association between oxytocin (OT) and pain. Methods: PsychInfo, PubMed, and Medline (EBSCO) research databases were searched for peer-reviewed articles written between 1950 and 2012. Of a total of 1166 articles returned, 50 (9 human, 33 animal, and 8 spinal cord samples) met full inclusion criteria and were included in the review. Results: OT had a reliable effect as defined by increasing pain tolerance in 29 of 33 animal studies reviewed. This effect persisted across central and peripheral modes of administration and type of noxious stimulus used (eg, heat, electric). The results suggest that OT acts as an analgesic for acute pain in animals. Preliminary research with humans offers consistent evidence to suggest that OT decreases pain sensitivity, though the reliability and stability of such effects cannot yet be determined. Although the findings are encouraging, there is a need for methodologically rigorous work in humans where OT is administered centrally. Discussion: Further research seems to be warranted as the existence of biologically and psychologically plausible mechanisms linking OT and pain have been well supported using animal models with limited but encouraging human research. Implications and recommendations are discussed. Findings from this research may inform therapeutic methods for the management of pain.
[1] Melis MR, Argiolas A. Central control of penile erection: a re-visitation of the role of oxytocin and its interaction with dopamine and glutamic acid in male rats. Neurosci Biobehav Rev. 2011;35:939–55.
[2] Hong SM, et al. Oxytocin: A potential therapeutic for obesity. J Obes Metab Syndr. 2021;30(2):115-23.
[3] Ohlsson B, et al. Oxytocin is expressed throughout the human gastrointestinal tract. Regul Pept. 2006;135:7–11.
[4] Yao S, Kendrick KM. Effects of Intranasal Administration of Oxytocin and Vasopressin on Social Cognition and Potential Routes and Mechanisms of Action. Pharmaceutics. 2022;14(2):323.
[5] Carter CS, et al. Is oxytocin “nature’s medicine” ? Pharmacol Rev. 2020;72(4):829-61.
[vi] Striepens N, et al. Elevated cerebrospinal fluid and blood concentrations of oxytocin following its intranasal administration in humans. Sci. Rep. 2013; 3: 3440.
[vii] Striepens N, et al. Elevated cerebrospinal fluid and blood concentrations of oxytocin following its intranasal administration in humans. Sci Rep. 2013;3:3440.
[viii] Witt DM, et al. Enhanced social interactions in rats following chronic, centrally infused oxytocin. Pharmacol Biochem Behav. 1992;43(3):855-61.
[ix] Lukas M, et al. The neuropeptide oxytocin facilitates pro-social behavior and prevents social avoidance in rats and mice. Neuropsychopharmacology. 2011;36(11):2159-68.
[x] Kosfeld M, et al. Oxytocin increases trust in humans. Nature. 2005;435(7042)673-6.
[xi] Gaustella AJ, et al. Oxytocin enhances the encoding of positive social memories in humans. Biol Psychiatr. 2008;64(3):256-8.
[xii] Domes G, et al. Oxytocin improves “mind-reading” in humans. Biol Psychiat. 2007;61(6):731-3.
[xiii] Kou J, et al. In the nose or on the tongue? Contrasting motivational effects of oral and intranasal oxytocin on arousal and reward during social processing. Translational Psychiatry. 2021;11:94.
[xiv] Kou J, et al. In the nose or on the tongue? Contrasting motivational effects of oral and intranasal oxytocin on arousal and reward during social processing. Translational Psychiatry. 2021;11:94.
[xv] Winslow JT, Insel TR: The social deficits of the oxytocin knockout mouse. Neuropeptides 2002;36:221–229.
[xvi] Born J, et al. Sniffing neuropeptides: a transnasal approach to the human brain. Nat Neurosci 2002;5:514–516.
[xvii] Striepens N, et al. Elevated cerebrospinal fluid and blood concentrations of oxytocin following its intranasal admiration in humans. Sci Rep. 2013;3:3440.
[xviii] Neumann ID, et al. Increased brain and plasma oxytocin after nasal and peripheral administration in rats and mice. Psychoneuroendocrinol. 2013;38(10):1985-93.
[xix] Guastella AJ, et al. Recommendations for the standardisation of oxytocin nasal administration and guidelines for its reporting in human research. Psychoneuroendocrinol. 2013;38(5):612-25.
[xx] Rosenbaum TY. Musculoskeletal pain and sexual function in women. J Sex Med. 2010 Feb;7(2 Pt 1):645-53.
[xxi] Caruso S, et al. Oxytocin plasma levels in orgasmic and anorgasmic women. Gynecol Endocrinol. 2018 Jan;34(1):69-72.
[xxii] Carmichael MS, et al. Plasma oxytocin increases in the human sexual response. J Clin Endocrinol Metab. 1987 Jan;64(1):27-31.
[xxiii] Caruso S, et al. Oxytocin plasma levels in orgasmic and anorgasmic women. Gynecol Endocrinol. 2018;34(1):69-72.
[xxiv] Behinia B, et al. Differntial effects of intranasal oxytocin on sexual experiences and partner interactions in couples. Horm Behav. 2014;65(3):308-18.
[xxv] Muin DA, et al. Effect of long-term intranasal oxytocin on sexual dysfunction in premenopausal and postmenopausal women: a randomized trial. Fertil Steril. 2015;104(3):715-23.
[xxvi] Kou J, et al. In the nose or on the tongue? Contrasting motivational effects of oral and intranasal oxytocin on arousal and reward during social processing. Translational Psychiatry. 2021;11:94.
[xxvii] Averbeck BB, et al. Emotion recognition and oxytocin in patients with schizophrenia. Psychol Med. 2012;42(2):259-66.
[xxviii] Shahrestani S, et al. The Impact of a Single Administration of Intranasal Oxytocin on the Recognition of Basic Emotions in Humans: A Meta-Analysis. Neuropsychopharmacol. 2013;38(10):1929-36.
[xxix] Gaustella AJ, et al. A randomized controlled trial of intranasal oxytocin as an adjunct to exposure therapy for social anxiety disorder. Psychoneuroendocrinol. 2009;34(6):917-23.
[xxx] Labuschagne I, et al. Oxytocin attenuates amygdala reactivity to fear in generalized social anxiety disorder. Neuropsychopharmacol. 2010;35(12):2403-13.
[xxxi] Scantamburlo G, et al. Plasma oxytocin levels and anxiety in patients with major depression. Psychoneuroendocrinology 2007;32(4):407–410.
[xxxii] Leckman JF, et al. Elevated cerebrospinal fluid levels of oxytocin in obsessive-compulsive disorder. Comparison with Tourette’s syndrome and healthy controls. Arch Gen Psychiatry 1994;51(10):782–792.
[xxxiii] Cacciotti-Saija C, et al. A Double-Blind Randomized Controlled Trial of Oxytocin Nasal Spray and Social Cognition Training for Young People With Early Psychosis. Scizophren Bull. 2015;41(2):483-93.
[xxxiv] Insel TR. The challenge of translation in social neuroscience: a review of oxytocin, vasopressin, and affiliative behavior. Neuron 2010; 65: 768–79.
[xxxv] Rutigliano G, Rocchetti M, Paloyelis Y, et al. Peripheral oxytocin and vasopressin: biomarkers of psychiatric disorders? A comprehensive systematic review and preliminary meta-analysis. Psychiatry Res 2016; 241: 207–20.
[xxxvi] LoParo D, Waldman ID. The oxytocin receptor gene (OXTR) is associated with autism spectrum disorder: a meta-analysis. Mol Psychiatry 2015; 20: 640–6.
[xxxvii] Gregory SG, Connelly JJ, Towers AJ, et al. Genomic and epigenetic evidence for oxytocin receptor deficiency in autism. BMC Med 2009; 7: 62.
[xxxviii] Freeman SM, Palumbo MC, Lawrence RH, Smith AL, Goodman MM, Bales KL. Effect of age and autism spectrum disorder on oxytocin receptor density in the human basal forebrain and midbrain. Transl Psychiatry 2018; 8: 257.
[xxxix] Sikich L, et al. Intranasal Oxytocin in Children and Adolescents with Autism Spectrum Disorder. N Eng J Med. 2021;385(16):1462-1473.
[xl] Watanabe T, Abe O, Kuwabara H, et al. Mitigation of sociocommunicational deficits of autism through oxytocin-induced recovery of medial prefrontal activity: a randomized trial. JAMA Psychiatry 2014; 71: 166–75.
[xli] Greene RK, Spanos M, Alderman C, et al. The effects of intranasal oxytocin on reward circuitry responses in children with autism spectrum disorder. J Neurodev Disord 2018; 10: 12.
[xlii] Anagnostou E, Soorya L, Chaplin W, et al. Intranasal oxytocin versus placebo in the treatment of adults with autism spectrum disorders: a randomized controlled trial. Mol Autism 2012;3:16.
[xliii] Watanabe T, Kuroda M, Kuwabara H, et al. Clinical and neural effects of six-week administration of oxytocin on core symptoms of autism. Brain 2015; 138: 3400–12.
[xliv] Wang Y, Wang M-J, Rong Y, He H-Z, Yang C-J. Oxytocin therapy for core symptoms in autism spectrum disorder: an updated meta-analysis of randomized controlled trials. Res Autism Spectr Disord 2019; 64: 63–75.
[xlv] Keech B, Crowe S, Hocking DR. Intranasal oxytocin, social cognition and neuro-developmental disorders: a meta-analysis. Psychoneuroendocrinology 2018; 87: 9–19.
[xlvi] Cai Q, et al. Systematic review and meta-analysis of reported adverse events of long-term intranasal oxytocin treatment for autism spectrum disorder. Psychiat Clin Neurosci. 2018;72(3):140-51.
[xlvii] González-Hernández A, Rojas-Piloni G, Condés-Lara M. Oxytocin and analgesia: future trends. Trends Pharmacol Sci 2014;35:549–51.
[xlviii] Rash JA, et al. Evaluating the efficacy of intranasal oxytocin on pain and function among individuals who experience chronic pain: a protocol for a multisite, placebocontrolled, blinded, sequential, withinsubjects crossover trial. BMJ Open. 2021;11:e055039.
[xlix] Gimpl G, Fahrenholz F. The oxytocin receptor system: structure, function, and regulation. Physiol Rev 2001;81:629–83.
[l] Rash JA, Aguirre-Camacho A, Campbell TS. Oxytocin and pain: a systematic review and synthesis of findings. Clin J Pain 2014;30:453–62.
[li] Tracy LM, Georgiou-Karistianis N, Gibson SJ, et al. Oxytocin and the modulation of pain experience: implications for chronic pain management. Neurosci Biobehav Rev 2015;55:53–67
[lii] Viero C, Shibuya I, Kitamura N, et al. Review: oxytocin: crossing the bridge between basic science and pharmacotherapy. CNS Neurosci Ther 2010;16:e138–56.
[liii] Jo YH, Stoeckel ME, Freund-Mercier MJ, et al. Oxytocin modulates glutamatergic synaptic transmission between cultured neonatal spinal cord dorsal horn neurons. J Neurosci 1998;18:2377–86.
[liv] Ge Y, Lundeberg T, Yu L-C. Blockade effect of mu and kappa opioid antagonists on the anti-nociception induced by intraperiaqueductal grey injection of oxytocin in rats. Brain Res 2002;927:204–7.
[lv] Yang J, Liang J-Y, Li P, et al. Oxytocin in the periaqueductal gray participates in pain modulation in the rat by influencing endogenous opiate peptides. Peptides 2011;32:1255–61.
[lvi] Miranda-Cardenas Y, Rojas-Piloni G, Martínez-Lorenzana G, et al. Oxytocin and electrical stimulation of the paraventricular hypothalamic nucleus produce antinociceptive effects that are reversed by an oxytocin antagonist. Pain 2006;122:182–9.
[lvii] Berna C, Leknes S, Holmes EA, et al. Induction of depressed mood disrupts emotion regulation neurocircuitry and enhances pain unpleasantness. Biol Psychiatry 2010;67:1083–90
[lviii] Rainville P, Bao QVH, Chrétien P. Pain-related emotions modulate experimental pain perception and autonomic responses. Pain 2005;118:306–18.
[lix] Heinrichs M, Baumgartner T, Kirschbaum C, et al. Social support and oxytocin interact to suppress cortisol and subjective responses to psychosocial stress. Biol Psychiatry 2003;54:1389–98.
[lx] Rash JA, Aguirre-Camacho A, Campbell TS. Oxytocin and pain: a systematic review and synthesis of findings. Clin J Pain 2014;30:453–62.
[lxi] Hong SM, et al. Oxytocin: a potential therapeutic for obesity. J Obes Metab Syndr. 2021;30(2):115-123.
[lxii] Ott V, Finlayson G, Lehnert H, Heitmann B, Heinrichs M, Born J, et al. Oxytocin reduces reward-driven food intake in humans. Diabetes. 2013;62:3418–25.
[lxiii] Lawson EA, Marengi DA, DeSanti RL, Holmes TM, Schoenfeld DA, Tolley CJ. Oxytocin reduces caloric intake in men. Obesity (Silver Spring) 2015;23:950–6.
[lxiv] Turton R, Chami R, Treasure J. Emotional eating, binge eating and animal models of binge-type eating disorders. Curr Obes Rep. 2017;6:217–28.
[lxv] Sabatier N, Leng G, Menzies J. Oxytocin, feeding, and satiety. Front Endocrinol (Lausanne) 2013;4:35.
[lxvi] Leslie M, et al. A Systematic Review and Quantitative Meta-Analysis of Oxytocin’s Effects on Feeding. J Neuroendocrinol. 2018;10.
[lxvii] Lawson EA. The effects of oxytocin on eating behaviour and metabolism in humans. Nat Rev Endocrinol. 2017;13:700–9.
[lxviii] Zhang H, Wu C, Chen Q, Chen X, Xu Z, Wu J, et al. Treatment of obesity and diabetes using oxytocin or analogs in patients and mouse models. PLoS One. 2013;8:e61477.
[lxix] Leslie M, Silva P, Paloyelis Y, Blevins J, Treasure J. A systematic review and quantitative meta-analysis of Oxytocin’s effects on feeding. J Neuroendocrinol. 2018;30:e12584.