Angiopoietin-related growth factor (AGF)
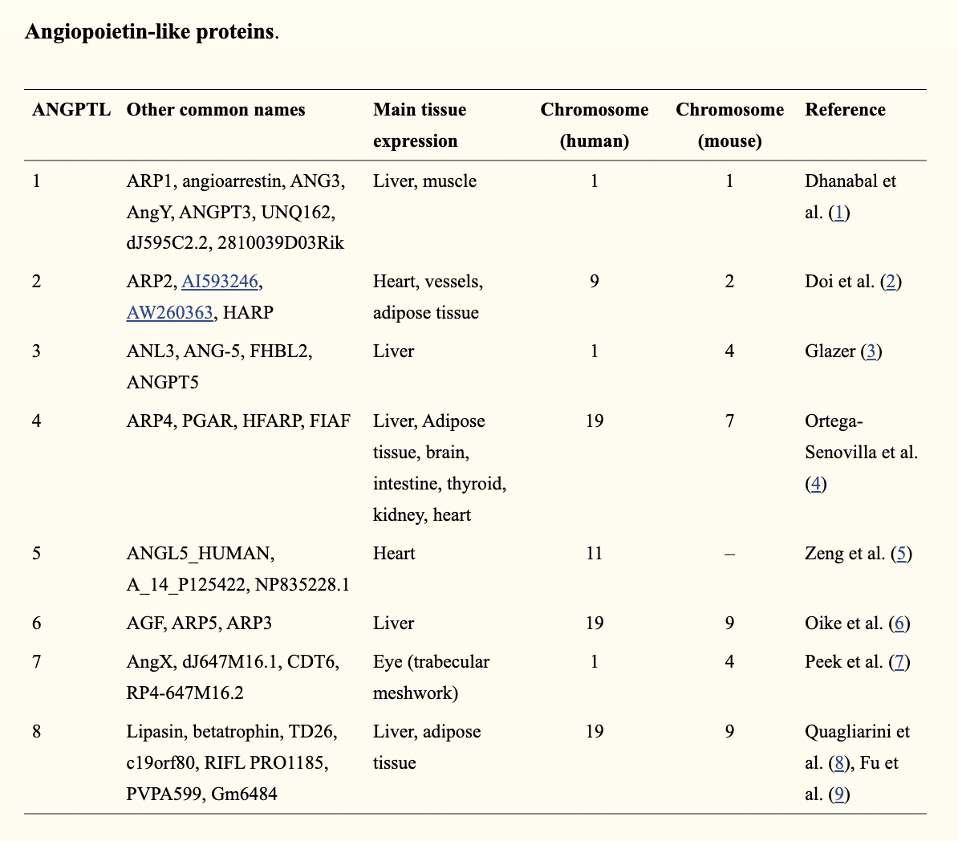
Angiopoietin-like (ANGPTL) proteins
ANGIOGENESIS
Contents
Most Frequent Uses:
- Vascular development
- Wound care/Skin issues
Dosage:
- None established
Safety and Potential Side Effects/Contraindications:
- Safety not established
Description
Angiopoietin-related growth factor (AGF), a member of the angiopoietin-like protein (Angptl) family, is secreted predominantly from the liver into the systemic circulation. AGFs are reported as positive mediators of angiogenesis, and antagonize insulin resistance and obesity.[i]
Angiopoietin-like proteins (ANGPTLs) are a family of proteins structurally similar to the angiopoietins. To date, eight ANGPTLs have been discovered, namely ANGPTL1 to ANGPTL8.
Various functions of ANGPTL proteins have also been described in developmental, physiological, and pathophysiological processes. Crucially, some ANGPTL proteins exhibit multibiological properties, including functional roles in lipid metabolism[ii], inflammation[iii], hematopoietic stem cell activity[iv], and cancer cell invasion.[v],[vi]
[i] Ebert T, et al. Serum levels of angiopoietin-related growth factor in diabetes mellitus and chronic hemodialysis. Metab Clin Exp. 2009;58:547-51.
[ii] Kersten S. Regulation of lipid metabolism via angiopoietin-like proteins. Biochem Soc Trans. 2005;33:1059–6210.
[iii] Tabata M, Kadomatsu T, Fukuhara S, Miyata K, Ito Y, Endo M, et al. Angiopoietin-like protein 2 promotes chronic adipose tissue inflammation and obesity-related systemic insulin resistance. Cell Metab. 2009;10:178–8810.
[iv] Zhang CC, Kaba M, Ge G, Xie K, Tong W, Hug C, et al. Angiopoietin-like proteins stimulate ex vivo expansion of hematopoietic stem cells. Nat Med. 2006;12:240–510.
[v] Galaup A, Cazes A, Le Jan S, Philippe J, Connault E, Le Coz E, et al. Angiopoietin-like 4 prevents metastasis through inhibition of vascular permeability and tumor cell motility and invasiveness. Proc Natl Acad Sci U S A. 2006;103:18721–610.
[vi] Kuo TC, Tan CT, Chang YW, Hong CC, Lee WJ, Chen MW, et al. Angiopoietin-like protein 1 suppresses SLUG to inhibit cancer cell motility. J Clin Invest. 2013;123:1082–9510.
Clinical Research
IPS Level of Evidence
IPS Clinical Pharmacists have developed a method of ranking the studies so that the practitioner can easily discern the level of evidence this study provides to the topic. Levels 1-8 are listed below:
| Level of Evidence | Description | |
| Level 1 | FDA Approved Drug studies | |
| Level 2 | Evidence obtained from systematic review and/or meta-analyses of studies including RCTs and other human studies | |
| Level 3 | Evidence obtained from a RCT | |
| X | Level 4 | Evidence obtained from a study without randomization |
| Level 5 | Evidence obtained from case reports | |
| X | Level 6 | Evidence obtained from in vitro human studies |
| X | Level 7 | Evidence obtained from laboratory animal studies |
| X | Level 8 | Evidence obtained from Opinions or Reviews |
Levels
Level 4
Mohasseb M, et al. Relationship between testosterone, sex hormone binding globulin, angiopoietin related growth factor and insulin resistance in normal weight and obese men. Alexandria J Med. 2014;50:37-44.
Abstract
Relationship between testosterone, sex hormone binding globulin, angiopoietin related growth factor and insulin resistance in normal weight and obese men. The present results revealed for the first time an association between SHBG and AGF serum levels. It could be suggested that they overlap to regulate metabolic homeostasis in normal weight men and that the disturbed inter-relationship could contribute to the pathogenesis of insulin resistance and obesity. Moreover, the observed relationship between SHBG and AGF in the present study could clarify the unresolved controversies regarding specific mechanistic relationships between SHBG abnormalities and abnormalities in glucose homeostasis.
Ebert T, et al. Serum levels of angiopoietin-related growth factor in diabetes mellitus and chronic hemodialysis. Metabolism. 2009;58(4):547-51.
Abstract
Angiopoietin-related growth factor (AGF) was recently introduced as a novel liver-derived protein that antagonizes obesity and insulin resistance. In the current study, we investigated circulating AGF levels in relation to renal function and type 2 diabetes mellitus (T2DM). Angiopoietin-related growth factor was determined by enzyme-linked immunosorbent assay in subjects with a glomerular filtration rate greater than 50 mL/min (n = 60, 30 diabetic and 30 nondiabetic) and in patients on chronic hemodialysis (CD; n = 60, 32 diabetic and 28 nondiabetic). Furthermore, AGF was correlated to clinical and biochemical measures of renal function, glucose and lipid metabolism, as well as inflammation.
Serum levels of angiopoietin-related growth factor in diabetes mellitus and chronic hemodialysis
Level 6
Ito Y, et al. Inhibition of angiogenesis and vascular leakiness by angiopoientin-related protein 4. Cancer Res. 2003;63(20):6651-7.
Abstract
Angiopoietins and angiopoietin-related proteins (ARPs) have been shown to regulate angiogenesis, a process essential for various neovascular diseases including tumors. Here, we identify ARP4/fasting-induced adipose factor/peroxisome proliferator-activated receptor gamma angiopoietin-related as a novel antiangiogenic modulatory factor. We hypothesized that ARP4 may regulate angiogenesis. In vitro experiments using purified recombinant ARP4 protein revealed that ARP4 markedly inhibited the proliferation, chemotaxis, and tubule formation of endothelial cells. Moreover, using corneal neovascularization and Miles permeability assays, we found that both vascular endothelial growth factor-induced in vivo angiogenesis and vascular leakiness were significantly inhibited by the addition of ARP4. Finally, we found remarkable suppression of tumor growth within the dermal layer associated with decreased numbers of invading blood vessels in transgenic mice that express ARP4 in the skin driven by the keratinocyte promoter. These findings demonstrate that ARP4 functions as a novel antiangiogenic modulatory factor and indicate a potential therapeutic effect of ARP4 in neoplastic diseases.
No Full Text Available
Level 7
Oike Y, et al. Angiopoietin-related growth factor (AGF) promotes epidermal proliferation, remodeling, and regeneration. 2003;100(16):9494-9499.
Abstract
We report here the identification of an angiopoietin-related growth factor (AGF). To examine the biological function of AGF in vivo, we created transgenic mice expressing AGF in epidermal keratinocytes (K14-AGF). K14-AGF mice exhibited swollen and reddish ears, nose and eyelids. Histological analyses of K14-AGF mice revealed significantly thickened epidermis and a marked increase in proliferating epidermal cells as well as vascular cells in the skin compared with nontransgenic controls. In addition, we found rapid wound closure in the healing process and an unusual closure of holes punched in the ears of K14-AGF mice. Furthermore, we observed that AGF is expressed in platelets and mast cells, and detected at wounded skin, whereas there was no expression of AGF detected in normal skin tissues, suggesting that AGF derived from these infiltrated cells affects epidermal proliferation and thereby plays a role in the wound healing process. These findings demonstrate that biological functions of AGF in epidermal keratinocytes could lead to novel therapeutic strategies for wound care and epidermal regenerative medicine.
Oike Y, et al. Angiopoietin-related growth factor (AGF) promotes epidermal proliferation, remodeling, and regeneration. Proc Natl Acad Sci USA. 2003;100(16):9494-9.
Abstract
We report here the identification of an angiopoietin-related growth factor (AGF). To examine the biological function of AGF in vivo, we created transgenic mice expressing AGF in epidermal keratinocytes (K14-AGF). K14-AGF mice exhibited swollen and reddish ears, nose and eyelids. Histological analyses of K14-AGF mice revealed significantly thickened epidermis and a marked increase in proliferating epidermal cells as well as vascular cells in the skin compared with nontransgenic controls. In addition, we found rapid wound closure in the healing process and an unusual closure of holes punched in the ears of K14-AGF mice. Furthermore, we observed that AGF is expressed in platelets and mast cells, and detected at wounded skin, whereas there was no expression of AGF detected in normal skin tissues, suggesting that AGF derived from these infiltrated cells affects epidermal proliferation and thereby plays a role in the wound healing process. These findings demonstrate that biological functions of AGF in epidermal keratinocytes could lead to novel therapeutic strategies for wound care and epidermal regenerative medicine.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC170946/pdf/1009494.pdf
Zhang CC, et al. Angiopoeitin-like proteins stimulate ex vivo expansion of hematopoietic stem cells. Nat Med. 2006;12(2):20-5.
Abstract
Successful ex vivo expansion of hematopoietic stem cells (HSCs) would greatly benefit the treatment of disease and the understanding of crucial questions of stem cell biology. Here we show, using microarray studies, that the HSC-supportive mouse fetal liver CD3(+) cells specifically express the proteins angiopoietin-like 2 (Angptl2) and angiopoietin-like 3 (Angptl3). We observed a 24- or 30-fold net expansion of long-term HSCs by reconstitution analysis when we cultured highly enriched HSCs for 10 days in the presence of Angptl2 or Angptl3 together with saturating levels of other growth factors. The coiled-coil domain of Angptl2 was capable of stimulating expansion of HSCs. Furthermore, angiopoietin-like 5, angiopoietin-like 7 and microfibril-associated glycoprotein 4 also supported expansion of HSCs in culture.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2771412/pdf/nihms-43569.pdf
Farahbakhshian E,. et al. Angiopoietin-like protein 3 promotes preservation of stemness during ex vivo expansion of murine hematopoietic stem cells. PLoS One. 2014;9(8):e105642.
Abstract
Allogeneic hematopoietic stem cell (HSC) transplantations from umbilical cord blood or autologous HSCs for gene therapy purposes are hampered by limited number of stem cells. To test the ability to expand HSCs in vitro prior to transplantation, two growth factor cocktails containing stem cell factor, thrombopoietin, fms-related tyrosine kinase-3 ligand (STF) or stem cell factor, thrombopoietin, insulin-like growth factor-2, fibroblast growth factor-1 (STIF) either with or without the addition of angiopoietin-like protein-3 (Angptl3) were used. Culturing HSCs in STF and STIF media for 7 days expanded long-term repopulating stem cells content in vivo by ∼6-fold and ∼10-fold compared to freshly isolated stem cells. Addition of Angptl3 resulted in increased expansion of these populations by ∼17-fold and ∼32-fold, respectively, and was further supported by enforced expression of Angptl3 in HSCs through lentiviral transduction that also promoted HSC expansion. As expansion of highly purified lineage-negative, Sca-1+, c-Kit+ HSCs was less efficient than less pure lineage-negative HSCs, Angptl3 may have a direct effect on HCS but also an indirect effect on accessory cells that support HSC expansion. No evidence for leukemia or toxicity was found during long-term follow up of mice transplanted with ex vivo expanded HSCs or manipulated HSC populations that expressed Angptl3. We conclude that the cytokine combinations used in this study to expand HSCs ex vivo enhances the engraftment in vivo. This has important implications for allogeneic umbilical cord-blood derived HSC transplantations and autologous HSC applications including gene therapy.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4149469/pdf/pone.0105642.pdf
Oike Y, et al. Angiopoetin-related growth factor antagonizes obesity and insulin resistance. Nat Med. 2005;11(4):400-8.
Abstract
Angiopoietin-related growth factor (AGF), a member of the angiopoietin-like protein (Angptl) family, is secreted predominantly from the liver into the systemic circulation. Here, we show that most of the AGF-deficient mice die at about embryonic day 13, whereas the surviving AGF-deficient mice develop marked obesity, lipid accumulation in skeletal muscle and liver, and insulin resistance accompanied by reduced energy expenditure relative to controls. In parallel, mice with targeted activation of AGF show leanness and increased insulin sensitivity resulting from increased energy expenditure. They are also protected from high-fat diet–induced obesity, insulin resistance and nonadipose tissue steatosis. Hepatic overexpression of AGF by adenoviral transduction, which leads to an approximately 2.5-fold increase in serum AGF concentrations, results in a significant body weight loss and increases insulin sensitivity in mice fed a high-fat diet. This study establishes AGF as a new hepatocyte derived circulating factor that counteracts obesity and related insulin resistance.
– Angiopoietin-related growth factor antagonizes obesity and insulin resistance.
Urano T, et al. Angiopoietin-Related Growth Factor Enhances Blood Flow Via Activation of the ERK1/2-eNOS-NO Pathway in a Mouse Hind-Limb Ischemia Model. Arteriosclerosis, Thrombosis and Vascular Biol. 2008;28(5):827-34.
Abstract
Objective— Transgenic mice overexpressing angiopoietin-related growth factor (AGF) exhibit enhanced angiogenesis, suggesting that AGF may be a useful drug target in ischemic disease. Our goal was to determine whether AGF enhances blood flow in a mouse hind-limb ischemia model and to define molecular mechanisms underlying AGF signaling in endothelial cells.
Methods and Results— Intramuscular injection of adenovirus harboring AGF into the ischemic limb increased AGF production, which increased blood flow through induction of angiogenesis and arteriogenesis, thereby reducing the necessity for limb amputation. In vitro analysis showed that exposing human umbilical venous endothelial cells to AGF increased nitric oxide (NO) production through activation of an ERK1/2-endothelial NO synthetase (eNOS) signaling pathway. AGF-stimulated eNOS phosphorylation, NO production, and endothelial cell migration were all abolished by specific MEK1/2 inhibitors. Moreover, AGF did not restore blood flow to ischemic hind-limbs of either mice receiving NOS inhibitor L-NAME or eNOS knockout mice.
Conclusion— Activation of an ERK1/2-eNOS-NO pathway is a crucial signaling mechanism by which AGF increases blood flow through induction of angiogenesis and arteriogenesis. Further investigation of the regulation underlying AGF signaling pathway may contribute to develop a new clinical strategy for ischemic vascular diseases.
We sought to determine whether AGF enhances blood flow in a mouse hind-limb ischemia model and to define its molecular mechanisms. Activation of an ERK1/2-eNOS-NO pathway is crucial for AGF-induced angiogenesis and arteriogenesis. AGF signaling pathway may contribute to develop a new clinical strategy for ischemic vascular diseases.
https://www.ahajournals.org/doi/epub/10.1161/ATVBAHA.107.149674
Level 8
Oike Y, et al. Angiopoietin-like proteins–potential therapeutic targets for metabolic syndrome and cardiovascular disease. Circ J. 2009;73(12):2192-7.
Abstract
Recent major increases in obesity and related metabolic diseases (known as the metabolic syndrome (MetS)) because of sedentary lifestyles and overnutrition in developed and developing countries, are an exploding medical and social problem. These conditions are associated with increased risk of cardiovascular disease (CVD), the leading cause of death. Thus, it is necessary to understand the molecular basis underlying MetS and develop effective preventive and therapeutic approaches against CVD. To date, 7 angiopoietin-like proteins (Angptls) that are structurally similar to angiopoietins have been identified. However, none binds to the angiopoietin receptor, Tie2, or to the closely related Tie1 receptor, suggesting that these ligands function differently from angiopoietins. Some Angptls potently regulate angiogenesis, similar to angiopoietins, whereas others have pleiotropic activity other than angiogenesis and function in lipid and energy metabolism. In this review, we focus on the roles of Angptl2 and Angptl6/angiopoietin-like growth factor (AGF) in the development of MetS and CVD, and discuss the potential for Angptl2 and Angptl6/AGF to function as molecular targets for the prevention and treatment of both conditions.
https://www.jstage.jst.go.jp/article/circj/73/12/73_CJ-09-0710/_pdf/-char/en
Hato T, et al. The Role of Angiopoietin-Like Proteins in Angiogenesis and Metabolism. Trends Cardio Med. 2008;18(1):6-14.
Abstract
Recently, a family of proteins structurally similar to the angiogenic regulating factors angiopoietins was identified and designated “angiopoietin-like proteins” (Angptls). Encoded by seven genes, Angptls 1 to 7 all possess an N-terminal coiled-coil domain and a C-terminal fibrinogen-like domain, both characteristic of angiopoietins. However, Angptls do not bind to either the angiopoietin receptor Tie2 or the related protein Tie1 and remain orphan ligands. Nonetheless, Angptls 1, 2, 3, 4, and Angptl6/angiopoietin-related growth factor function to regulate angiogenesis. Angptls 3, 4, and Angptl6/angiopoietin-related growth factor also appear to directly regulate lipid, glucose, and energy metabolism independently of angiogenic effects. Recently, several lines of evidence reveal differential roles of Angptl structural domains in both angiogenesis and metabolism. Here, we briefly review what is currently known about Angptls function.